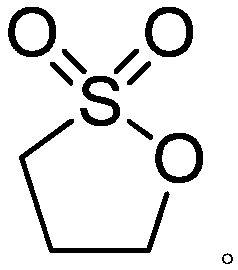
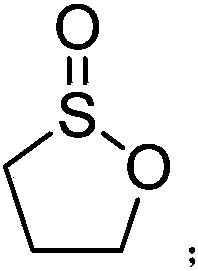
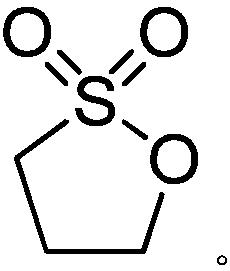
Preparation method of 1,3-propane sultone
A technology of propane sultone and propane sultone is applied in the field of preparation of 1,3-propane sultone, can solve the problems of flammability, explosion, strong corrosiveness, environmental hazards, etc. Inexpensive and readily available, mild reaction conditions and ideal yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Add 450 g of anhydrous acetonitrile, 1,3-dichloropropane (113 g, 1 mol) and tetraethylammonium bromide (10 g, 0.05 mol) into a 1 L four-necked flask connected with a glassy carbon electrode, and feed a constant current. The density is 100mA / cm 2 After being heated to 40°C, 128g of dry sulfur dioxide gas is slowly passed through for 8 hours, and the tail gas is absorbed with liquid caustic soda. Gas chromatographic tracking of the reaction process, the product content unchanged after the ventilation is over, the reaction is over, concentrated acetonitrile under reduced pressure, added 400g of dichloromethane to dissolve the concentrated solution, washed twice with 100g of water, dried over anhydrous magnesium sulfate, concentrated to give 1,3 - 42 g of propane sultone, yield 39.6%, purity 99.4%.
[0031] product: 1 H-NMR (CDCl 3 ): δ(ppm)1.82(m,-CH 2 -), 2.57(t, J=6.3Hz, CH 2 S), 4.42(t, J=5.8Hz, CH 2 O)
[0032] Add 42g of 1,3-propane sultone, 100g of dichloromet...
Embodiment 2
[0035] Add 450 g of anhydrous acetonitrile, 1,3-dichloropropane (113 g, 1 mol) and tetraethylammonium bromide (10 g, 0.05 mol) into a 1 L four-necked flask connected with a glassy carbon electrode, and feed a constant current. The density is 100mA / cm 2 After being heated to 80°C, 96g of dry sulfur dioxide gas is slowly passed through for 6 hours, and the tail gas is absorbed with liquid caustic soda. Gas chromatographic tracking reaction process, product content constant after ventilating finishes, reaction finishes, acetonitrile is concentrated under reduced pressure, adds 200g dimethyl carbonate to dissolve concentrated solution, 50g water washes twice, anhydrous magnesium sulfate is dried, concentrates after filtering and obtains 1, 90 g of 3-propane sultone, yield 84.9%, purity 99.1%.
[0036] Add 90g of 1,3-propane sultone and 300g of dichloromethane into a 500mL three-necked flask, slowly add 160g of hydrogen peroxide with a mass fraction of 20% at room temperature, and...
Embodiment 3
[0038] Add 450 g of anhydrous acetonitrile, 1,3-dichloropropane (113 g, 1 mol) and tetraethylammonium bromide (10 g, 0.05 mol) into a 1 L four-necked flask connected with a glassy carbon electrode, and feed a constant current. The density is 100mA / cm2 After being heated to 80°C, 64g of dry sulfur dioxide gas is slowly passed through for 6 hours, and the tail gas is absorbed with liquid caustic soda. Gas chromatography traced the reaction process, and the product content remained unchanged after aeration. After the reaction was completed, the acetonitrile was concentrated under reduced pressure, and 200 g of dimethyl carbonate was added to dissolve the concentrate, washed twice with 100 g of water, dried over anhydrous magnesium sulfate, and concentrated to obtain 1,3 - Propylene sultone 89g, yield 83.9%, purity 99.2%.
[0039] Add 89g of 1,3-propane sultone and 200g of dimethyl carbonate into a 500mL three-neck flask, slowly add 157g of hydrogen peroxide with a mass fraction o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com