Pyrene-based ratiometric fluorescent probe as well as preparation method and biological application thereof
A fluorescent probe, ratiometric technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of high detection limit and poor photostability of fluorescent probes, and achieve low detection limit and time Short, good photostability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] Example 1: A ratiometric fluorescent probe based on pyrene, which is 1-(pyrene-1-yl)-3-(4,5-dibromothiophen-2-yl)propenone, its chemical The structural formula is as follows: .
[0022] The method for preparing the ratiometric fluorescent probe based on pyrene, using pyrene as a starting material, reacting with anhydrous aluminum trichloride and acetyl chloride according to a molar ratio of 4:5:7 to obtain 1-acetylpyrene, Mix and dissolve the obtained 1-acetylpyrene and 4,5-dibromothiophene-2-carbaldehyde in a molar ratio of 3:4, then add 10% NaOH solution and stir at room temperature to obtain 1-(pyrene-1 -yl)-3-(4,5-dibromothien-2-yl)propenone.
[0023] Specific steps are as follows:
[0024] (1) Synthesis of 1-acetylpyrene: Add 1.00g (4mmol) pyrene and 40mL dichloromethane to a dry 100mL single-necked round-bottom flask equipped with magnetic stirring, stir to dissolve (the solution turns light yellow), and then Quickly add about 0.67g (5mmol) of anhydrous alumi...
Embodiment 2
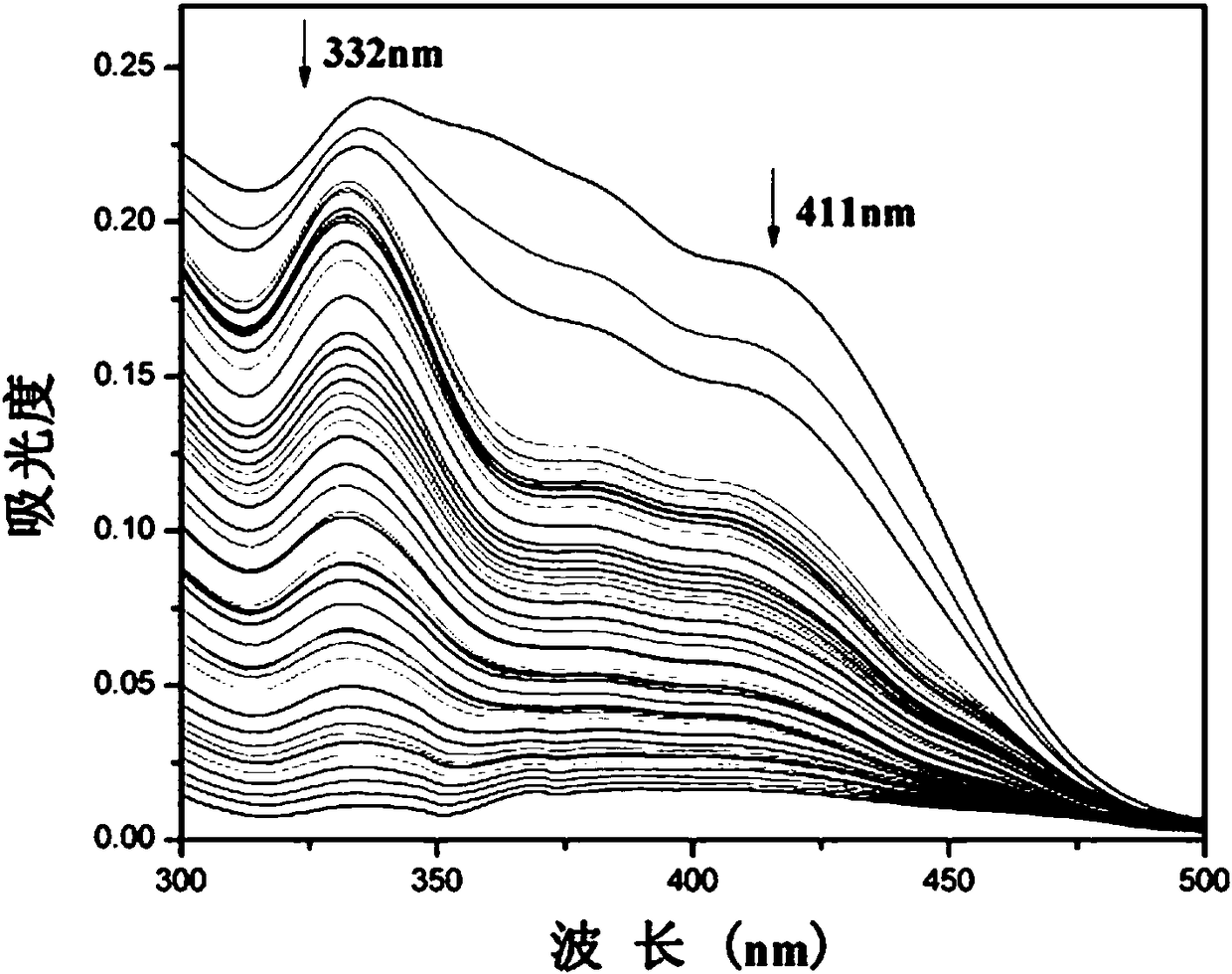
[0028] Example 2 HSO of fluorescent probe 3 - Varying UV Absorption Spectrum
[0029] Add 20.0 µL fluorescent probe stock solution to 2.0 mL water / absolute ethanol (9 / 1, v / v) system for HSO 3 - UV titration experiment, and record its UV absorption spectrum ( figure 1 ). With HSO 3 - The UV absorption at 332 nm and 411 nm both decreased with the increase of the amount.
Embodiment 3
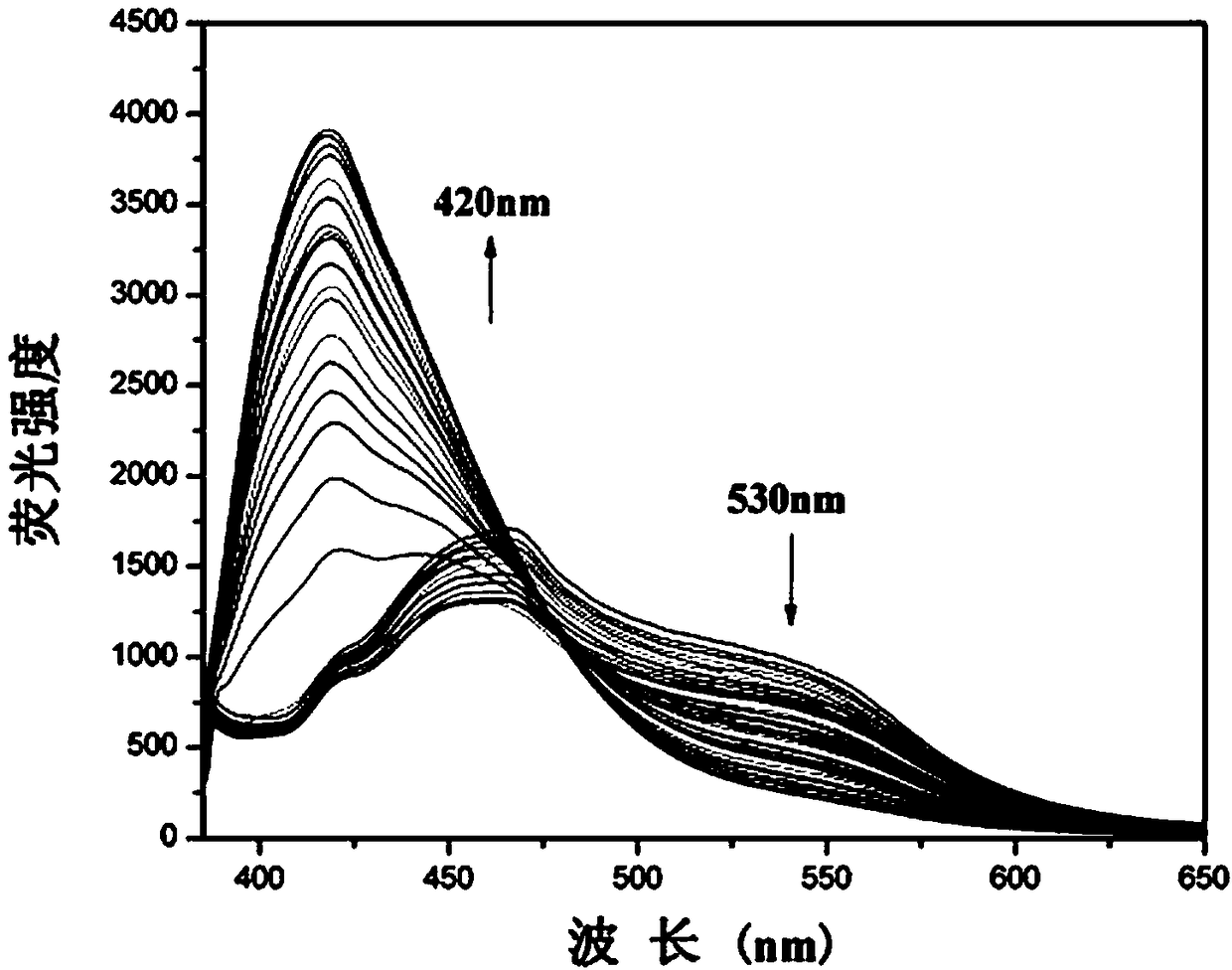
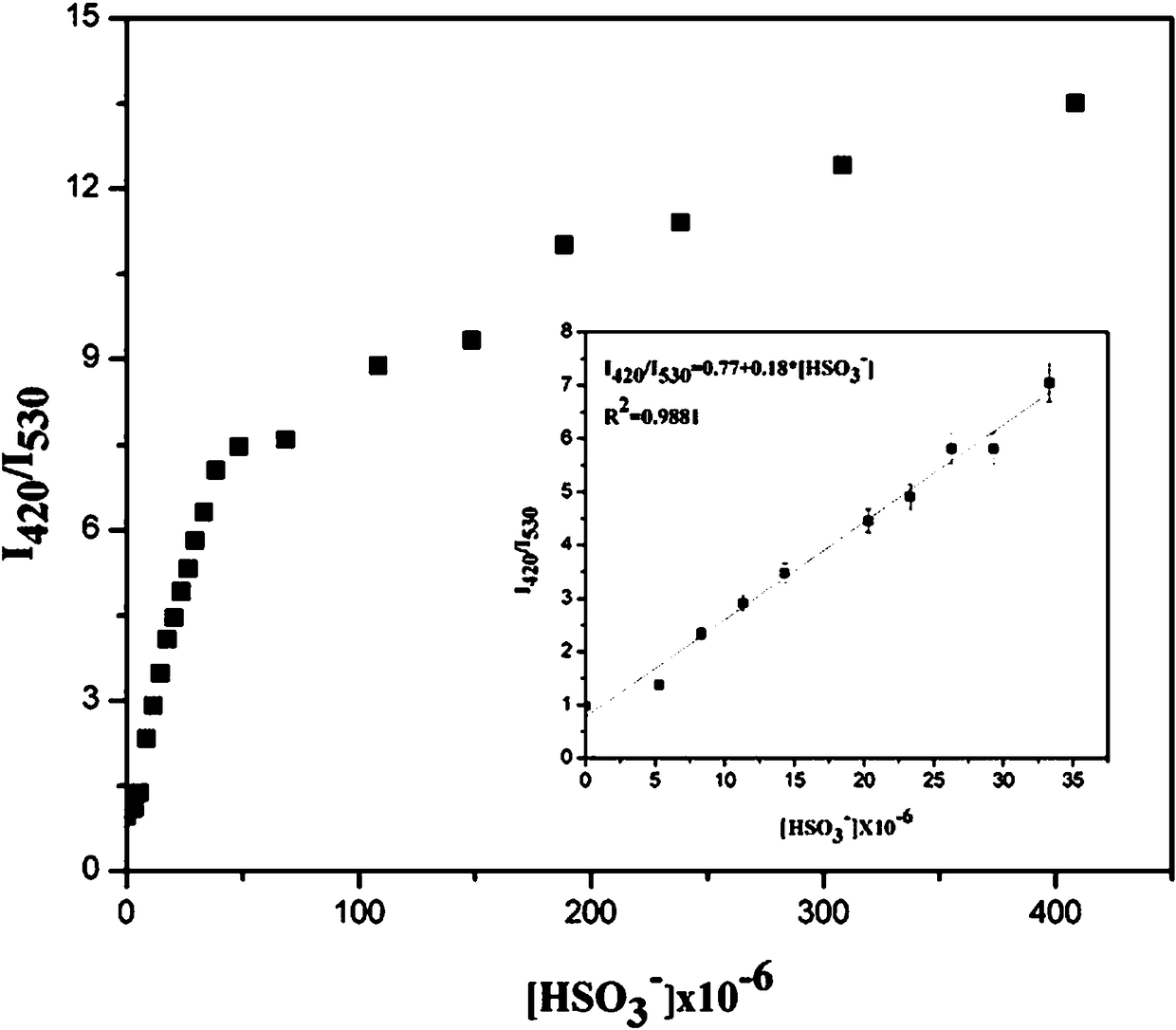
[0030] Embodiment 3 Fluorescent probes with HSO 3 - Fluorescence titration plot of change
[0031] Add 20.0 µL fluorescent probe stock solution to 2.0 mL water / absolute ethanol (9 / 1, v / v) system for HSO 3 - Fluorescent titration experiment, detected on a spectrofluorometer, along with HSO 3 - With the increase of , the fluorescence intensity at 530nm gradually weakened, and a new peak appeared at 420nm and gradually increased ( figure 2 ). Instrument parameters: The slit widths of the excitation wavelength and emission wavelength are 5.0 nm and 10 nm respectively, the voltage is 600 V, and the maximum excitation wavelength of the fluorescent probe solution is: λ ex is 366nm and the maximum emission wavelength is λ em 530nm. Fluorescence ratio I 420 / I 530 Plotting the graph for the ordinate gives the HSO 3 - The working curve of concentration, linear regression equation is: I 420 / I 530 = 0.18*[HSO 3 - ]+0.77, HSO 3 - The unit of concentration is 10 -6 mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Linear correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com