Quinoline triazole rare-earth compound as well as preparation method and application thereof
A technology of rare earth complexes and quinoline triazoles, which is applied in the fields of chemical instruments and methods, semiconductor/solid-state device manufacturing, electrical components, etc., and can solve the problems of poor stability and light stability of rare earth complexes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
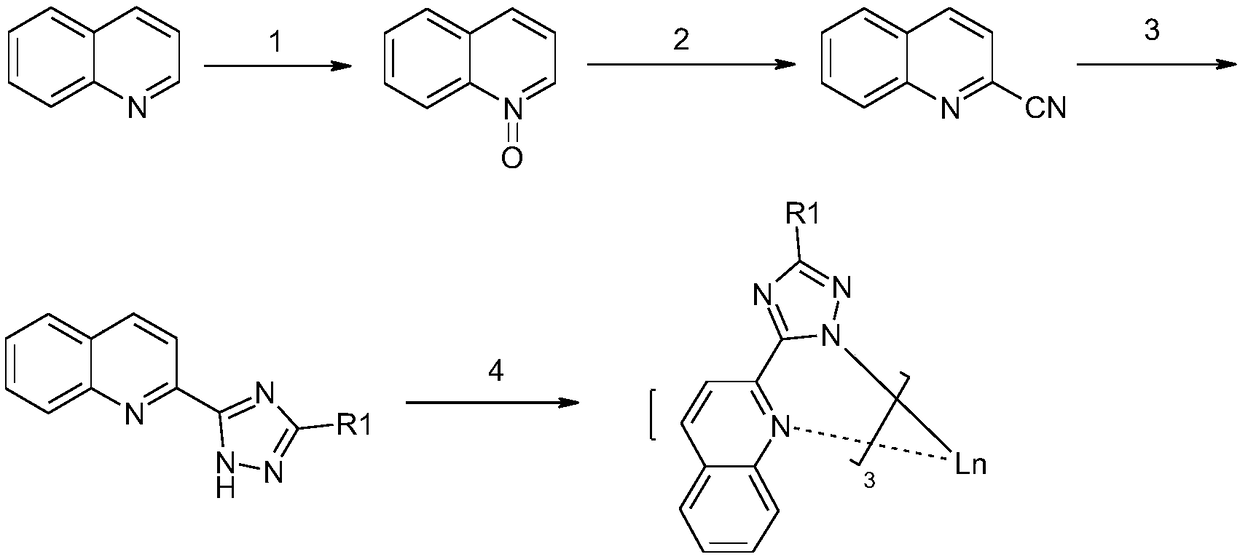
[0042] The preparation method of the quinolinetriazole rare earth complex comprises the following steps:
[0043] 1) Oxidation of quinoline to nitrogen oxides
[0044] 2) Nitrogen oxide N Substituting O with cyano groups gives 2-cyano-N-quinolines
[0045] 3O) 2N-cyanoquinoline Hydrazine reacts with carboxylic acid or carboxylic acid derivative N to give ligand compound
[0046]
[0047] 4) reacting the ligand compound with the rare earth metal salt to obtain the quinolinetriazole rare earth complex with the structure shown in formula (1).
[0048] The synthetic route diagram of this quinoline triazole rare earth complex preparation method is shown in the appendix figure 1 .
[0049] Preferably, in step 1) of the preparation method, specifically adding an oxidizing agent to perform an oxidation reaction; the oxidizing agent used in the oxidation reaction is at least one of m-chloroperoxybenzoic acid and hydrogen peroxide; the molar ratio of the oxidizing agent to...
Embodiment 1
[0071] Synthesis of tris[5-(quinolin-2-yl)-1,2,4-1H-triazole]europium(III)(compound 5)
[0072] The first step: the preparation of N-oxo-quinoline (compound 2)
[0073]
[0074] First, quinoline (compound 1, 129g) and acetic acid (600mL) were added into a 2L three-necked flask, stirred evenly, and the mass fraction of adding 30% H 2 o 2 (100mL), heated to 70-75°C, stirred for 3 hours, cooled to room temperature, added mass fraction of 30% H 2 o 2 (100mL), continue heating to 60-110°C, and react for 3 hours. After cooling to room temperature, the acetic acid was concentrated under reduced pressure to obtain a red viscous oil, which was diluted with water (1000 mL), and the pH was adjusted to 8-9 with solid sodium carbonate. The resulting solution was extracted with dichloromethane (1000 mL+500 mL×3), and the organic phases were combined and dried with anhydrous sulfuric acid. After filtration, the filtrate was concentrated under pressure to obtain an orange-red oil, whi...
Embodiment 2
[0089] Preparation of tris[5-(quinolin-2-yl)-1,2,4-1H-triazole]samarium(III)(compound 6)
[0090]
[0091] Compound 4 (5.9g) and samarium trichloride hexahydrate (3.7g) obtained in Example 1 were dissolved in 50mL of 2-ethoxyethanol:water (V:V)=1:3 mixed solvent , dubbed solutions C and D. Add 1.2g of sodium hydroxide to solution C, and stir for half an hour. Then, the D solution was added dropwise into the reaction bottle of the C solution, and the reaction was stirred at room temperature for 8 hours. After the reaction was completed, the solvent was evaporated to dryness under reduced pressure, and the solid was vacuum-dried at 50° C. for 3 hours to obtain 6.5 g of gray-yellow powder.
[0092] MS: [M+1] 735.9, SmC 33 h 21 N 12 M.W.=735, M+H peak 735.9 and M+Na peak 758.1 were detected.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap