Preparation method and application of a kind of terpyridine compound and metal complex thereof
A technology of metal complexes and terpyridines, applied in the direction of zinc organic compounds, chemical instruments and methods, applications, etc., can solve the problems of short duration, light stimulus response, transmission, etc., and achieve the effect of mild conditions and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
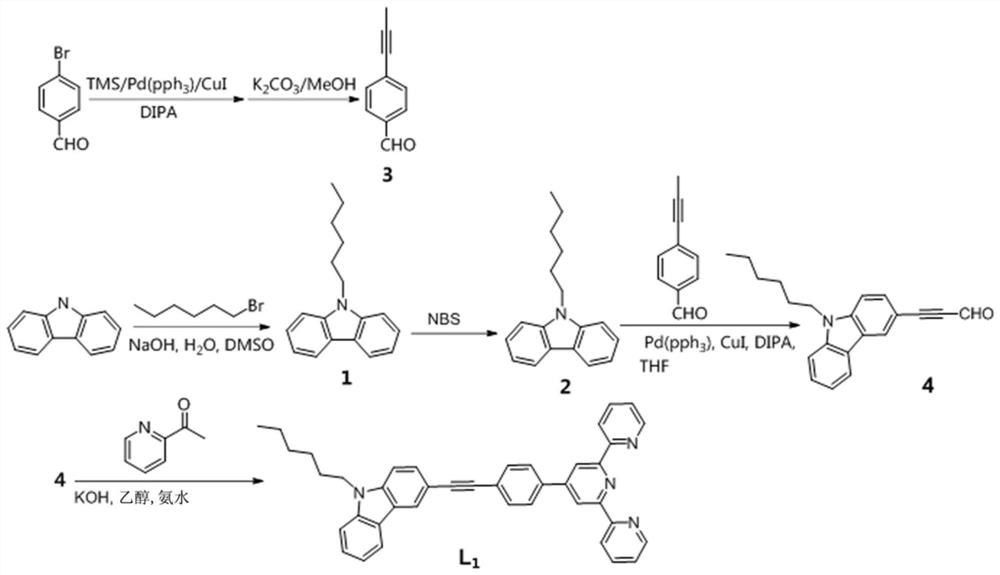
[0035] Embodiment 1: Terpyridine compound L 1 preparation
[0036] Synthetic route such as figure 1 As shown, the specific steps are as follows:
[0037] Step 1, in the DMSO solvent system, react carbazole, bromohexane and 50% sodium hydroxide aqueous solution in a molar ratio of 1:2:2 at 50°C for 8 hours, first distill under reduced pressure, extract with water and ethyl acetate, and obtain The organic layer was then washed with anhydrous Na 2 SO 4 Dried, spin-dried, separated and purified to obtain compound 1, its structural formula is:
[0038]
[0039] Step 2, in a dichloromethane solvent system, react compound 1 and NBS at a molar ratio of 1.2:1 at 25°C for 8 hours, filter, and wash with dichloromethane several times to obtain compound 2, whose structural formula is:
[0040]
[0041] Step 3, under the condition of nitrogen protection, in the dry diisopropylamine solution system, p-bromobenzaldehyde, trimethylethynyl silicon, cuprous iodide and tetrakistripheny...
Embodiment 2
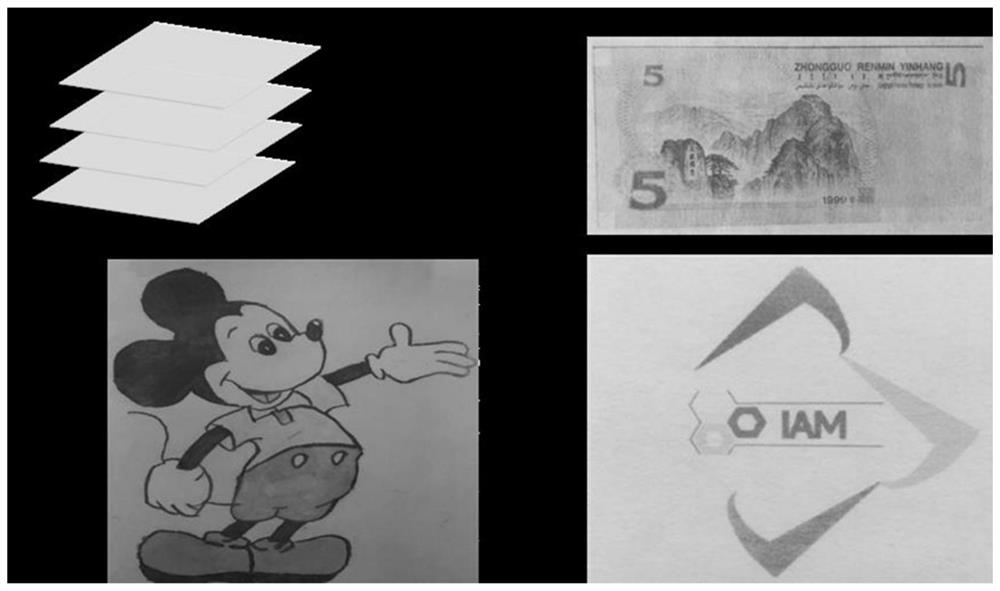
[0053] Embodiment 2: the preparation of multi-color copying paper
[0054] (1) Coat the polyethylene glycol-polypropylene glycol-polyethylene glycol (PEG-PPG-PEG) solution that is dissolved in dichloromethane on the filter paper, dry, as the first layer;
[0055] (2) The terpyridine compound L dissolved in dichloromethane 1 Apply the PEG-PPG-PEG mixture evenly on the first layer as the second layer;
[0056] (3) Coat the second layer with PEG-PPG-PEG as the third layer to make multi-color copy paper.
Embodiment 3
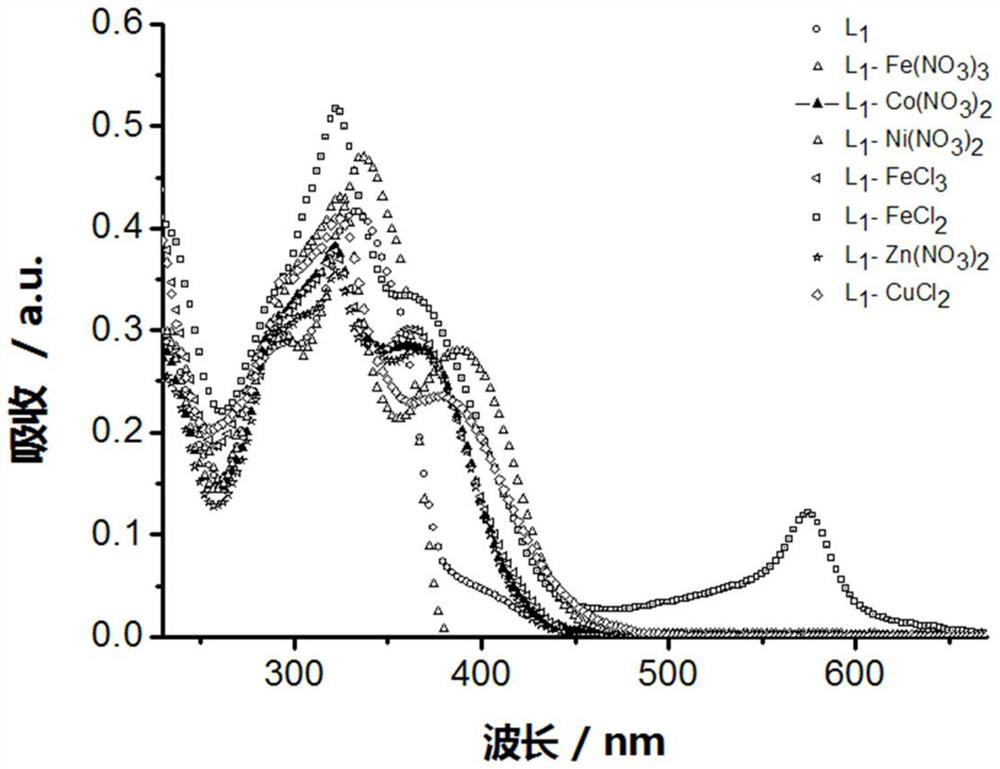
[0057] Example 3: Terpyridine Metal Complex L 1 -M preparation
[0058] A luminescent terpyridine metal complex, the structure consists of two parts: one part is terpyridine compound L 1 , and the other part is a different metal salt.
[0059] Terpyridine Metal Complex L 1 -Synthesis of M: the metal salt is directly combined with the corresponding terpyridine compound L 1 Stir in methanol solution at room temperature for 2h. L 1 The general structural formula of -M is as follows:
[0060]
[0061] Where M=Fe(NO 3 ) 3 , Co(NO 3 ) 2 , Ni(NO 3 ) 2 , FeCl 2 , FeCl 3 , CuCl 2 , Zn(CH 3 COO) 2 , ZnCl 2 , Zn(ClO 4 ) 2 and Zn(NO 3 ) 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com