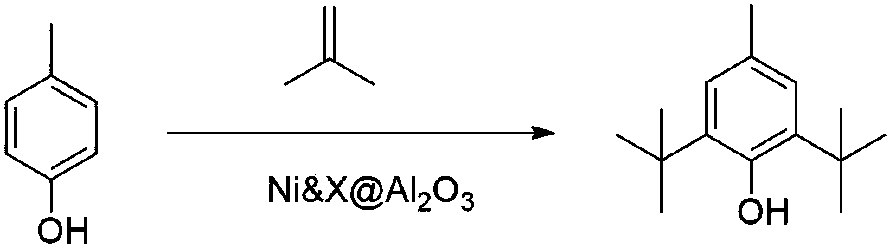
Preparation method of antioxidant BHT
A technology of antioxidants and synthesis methods, applied in the preparation of organic compounds, chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, etc., to improve thermal stability, improve preparation efficiency, and promote catalytic activity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-18
[0032] The method of preparing a nickel-doped activated alumina solid catalyst by the sol-gel method is as follows: Weigh a calculated amount of nickel compound and group VIII transition metal salt compound into ethylene glycol / butanol, and then add it to ethylene glycol / butanol at room temperature. Aluminum isopropoxide is added to the continuously stirred solution, and the resulting solution is allowed to stand and age for 24 to 120 hours. The obtained gel is evaporated and concentrated. A ball mill is used to prepare pellets with a diameter of 1 to 2 mm. After drying at room temperature, they are dried at 110 to 120°C for 12 to 36 hours, and then calcined in air at 400 to 600°C for 4 to 6 hours. .
[0033] Weigh 15g each of the nickel-doped activated alumina catalysts CUI-1~CUI-18 prepared by the sol-gel method in batches and fill them in the constant temperature section of the fixed bed reactor, and fill both ends of the catalyst bed Inert magnetic ball. The reactor is divi...
Embodiment 19-24
[0037] Weigh 15g of CUI-16 and fill it in the constant temperature section of the fixed bed reactor. The two ends of the catalyst bed are filled with inert magnetic balls. The reactor is divided into four sections for temperature control. There is a built-in thermocouple in the middle of the reactor for real-time Monitor the actual temperature at each point of the catalyst bed. Before the reaction, the temperature of the gasification chamber was gradually increased to 350°C under nitrogen flow, and the temperature of the catalyst bed was gradually increased to 230-260°C. After reaching the set temperature, continue to purge with nitrogen for 0.5 hours. The p-cresol is melted by heating with a heating belt, and then the mass space velocity is controlled to 0.1~2h with a syringe pump -1 . The isobutylene corresponding to 1:1~1:3 molar equivalent is delivered to the gasification chamber with a volume mass flow meter. After the p-cresol is gasified in the gasification chamber, it...
Embodiment 25
[0041] Using the catalyst CUI-16, change isobutylene to tert-butanol according to the experimental method in the above examples, control the molar ratio of tert-butanol to p-cresol to 2.8, mix tert-butanol and p-cresol evenly, and then inject the sample. The temperature of the layer was controlled at 260°C, and after two hours of continuous reaction, the samples were taken within 15 minutes and analyzed by gas chromatography. The conversion rate of p-cresol was 81% and the selectivity of BHT was 82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com