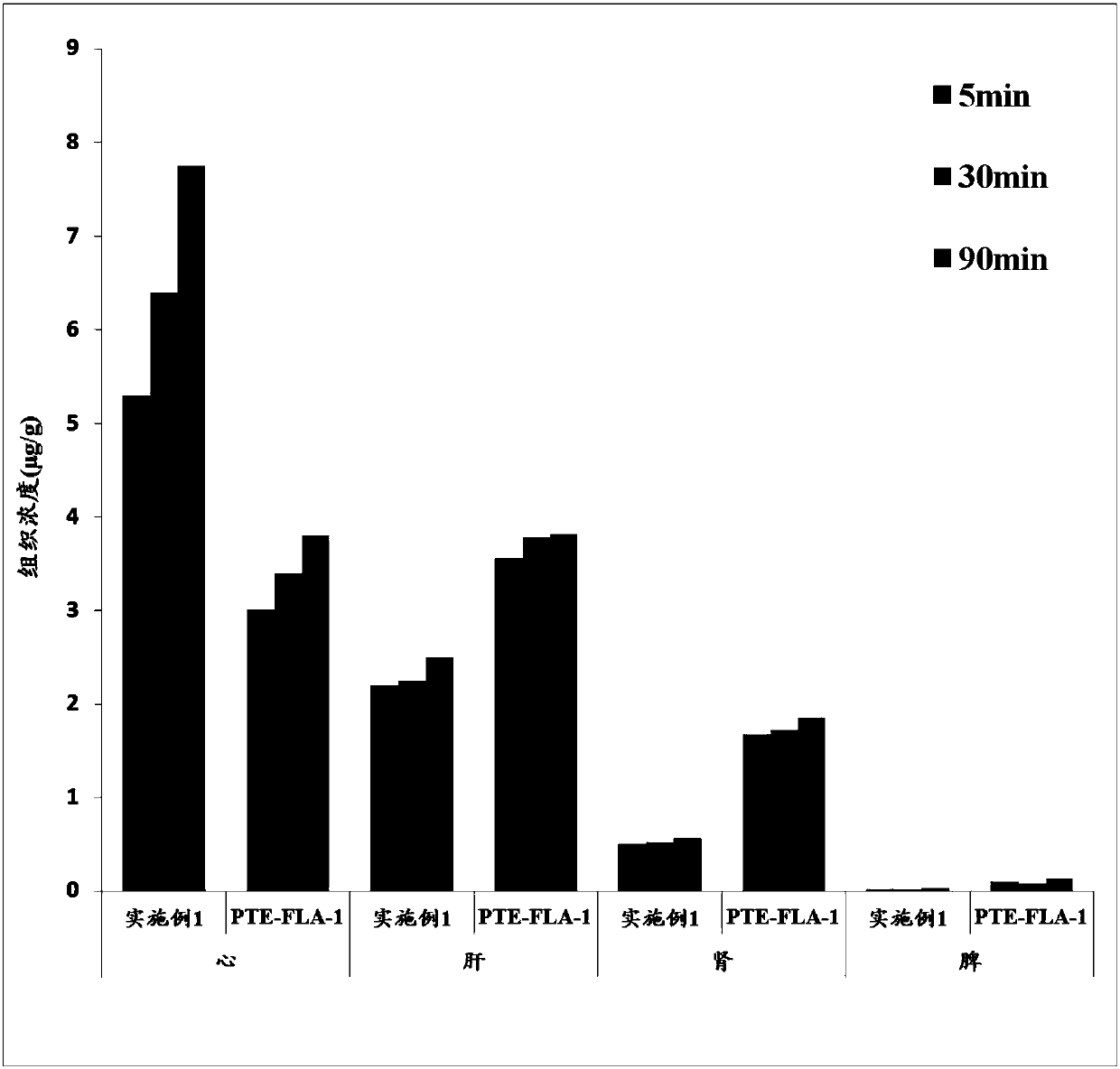
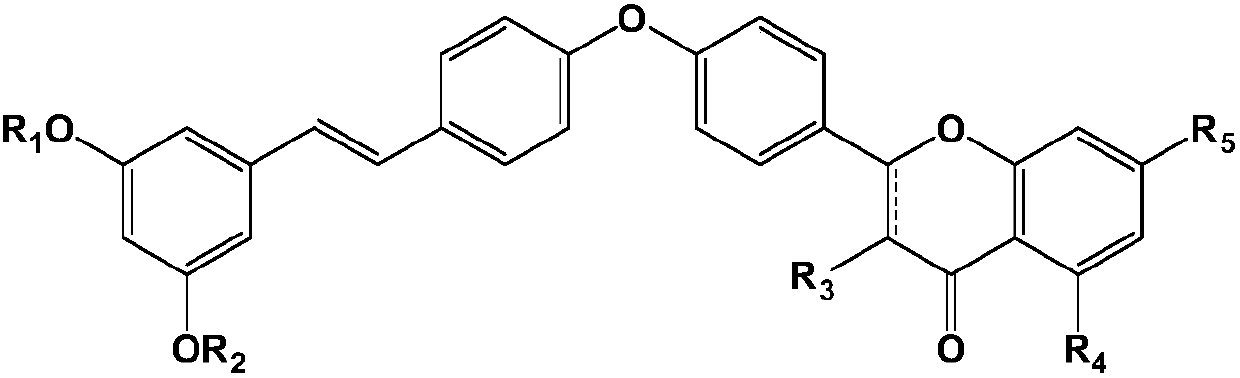
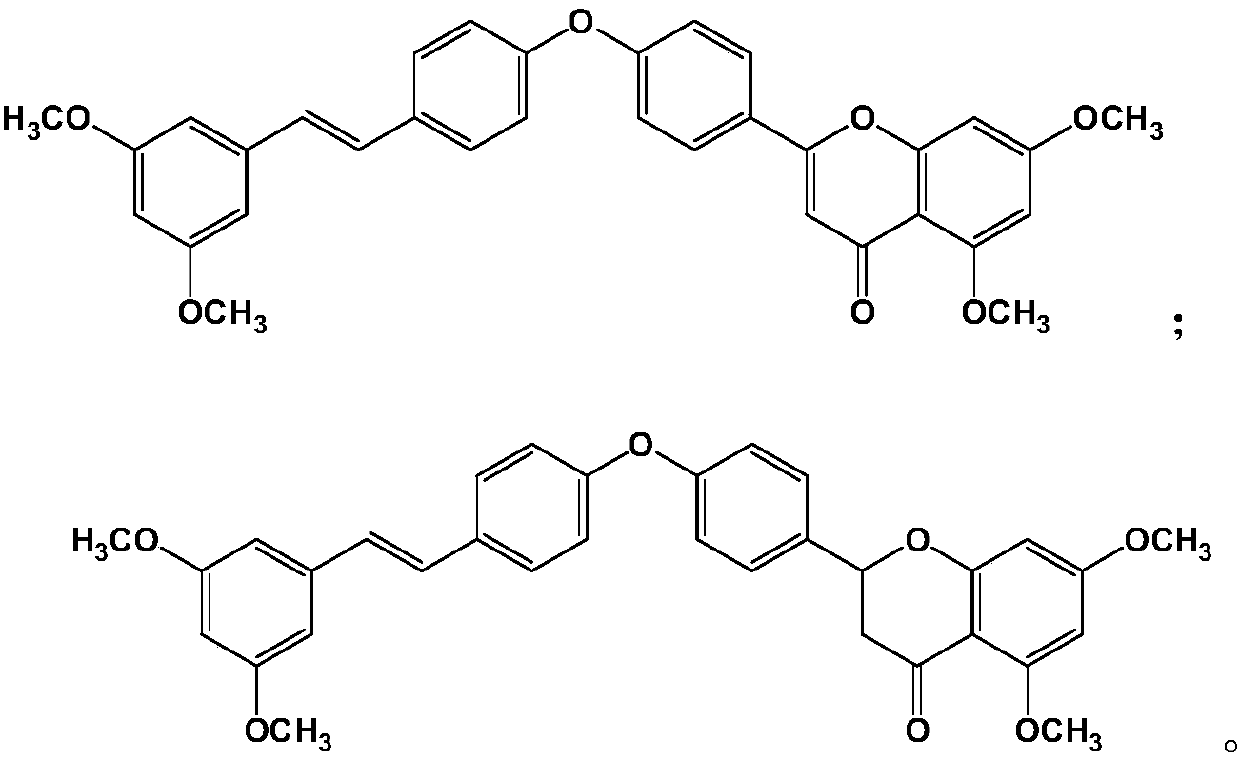
Pterostilbene compound myocardium targeted preparation and application thereof
A technology of pterostilbene and its compounds, which is applied in the field of medicine, can solve the problems that the drug tissue specificity is difficult to reach the lesion site, easy to degrade and unstable, etc., and achieve the effects of reducing drug dosage, simple and easy preparation method, and reducing drug concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Synthesis Example 1: (E)-2-(4-(4-(3,5-dimethoxystyryl)phenoxy)phenyl)-5,7-dimethoxy-4H-benzene Synthesis of pyran-4-one (PTE-FLA-1)
[0059]
[0060] Dissolve pterostilbene (5mmol) and potassium carbonate (10mmol) in 50ml of ethanol at 50°C, then add 2-(4-chlorophenyl)-5,7-dimethoxy-4H-benzopyran -4-ketone (5mmol), the system was heated to reflux, and stirred for 7 hours under reflux. After the reaction was monitored by TLC, it was cooled to room temperature, insolubles were removed by filtration, ethanol was removed by rotary evaporation, and the residue was separated by silica gel column chromatography, using ethyl acetate / petroleum ether=1:5 as eluent, and the target product The liquid was evaporated to remove the solvent, and heated and dried under vacuum to obtain 2.2 g of the target compound as a white solid, with a yield of 83.4%.
[0061] Elemental analysis: theoretical value / measured value, C(73.87 / 74.03), H(5.26 / 5.11), O(20.87 / 20.86)
[0062] ESI-MS: 537...
Embodiment 2
[0064] Synthesis Example 2: (E)-2-(4-(4-(3,5-dimethoxystyryl)phenoxy)phenyl)-5,7-dimethoxy-benzopyridine Synthesis of Pyran-4-one (PTE-FLA-2)
[0065]
[0066] Dissolve pterostilbene (5mmol) and potassium carbonate (10mmol) in 50ml of ethanol at 50°C, then add 2-(4-chlorophenyl)-5,7-dimethoxy-benzopyran-4 - Ketone (5mmol), the system was heated to reflux, and stirred for 8 hours under reflux. After the reaction was monitored by TLC, it was cooled to room temperature, the insoluble matter was removed by filtration, ethanol was removed by rotary evaporation, and the residue was separated by silica gel column chromatography, using ethyl acetate / cyclohexane=1:5 as eluent, and collected The liquid product was evaporated to remove the solvent, and dried under vacuum to obtain 2.1 g of the target compound as a white solid with a yield of 79.5%.
[0067] Elemental analysis: theoretical value / measured value, C(73.59 / 73.39), H(5.61 / 5.73), O(20.79 / 20.88)
[0068] ESI-MS: 539[M+H] ...
Embodiment 3
[0070] Synthesis Example 3: Synthesis of Histidine-modified Vitamin E Polyethylene Glycol Succinate (TPGS-His)
[0071]
[0072] 60g TPGS and 17.5g Boc-His were dissolved in 300ml of pyridine, then 18g EDC·HCl and 12.7g HOBT were added, stirred at room temperature for 10h, and the reaction was monitored by TLC. After the reaction was completed, the solvent was removed by rotary evaporation under reduced pressure, and the residual The product was separated by silica gel column chromatography with MeOH / petroleum ether (1:20) as eluent to obtain TPGS-His-Boc. Dissolve it in ethanol again, pass through dry HCl gas, neutralize and wash with NaOH after the reaction is completed, remove the solvent by rotary evaporation under reduced pressure, the residue is separated by silica gel column chromatography, and MeOH / petroleum ether (1:10) is used as The eluent finally obtained TPGS-His 28.5g.
[0073] Histidine-modified polyethylene glycol 12-hydroxystearate (HS-15-His), tryptophan-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com