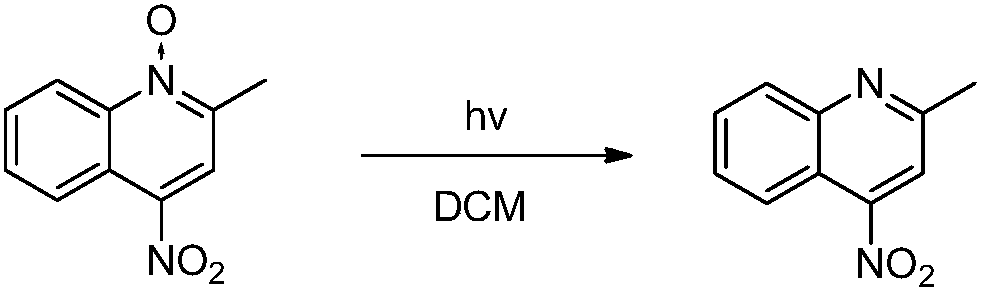
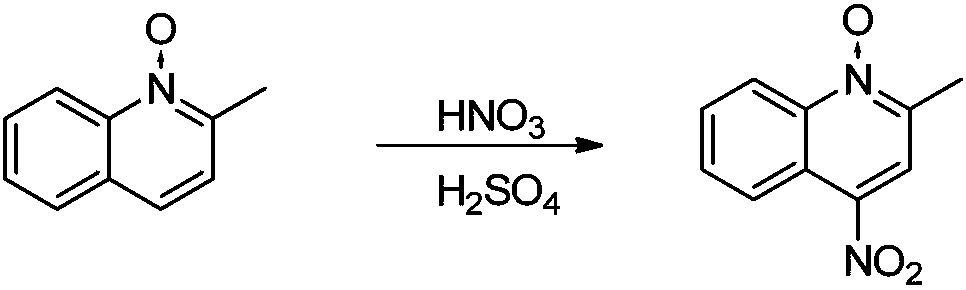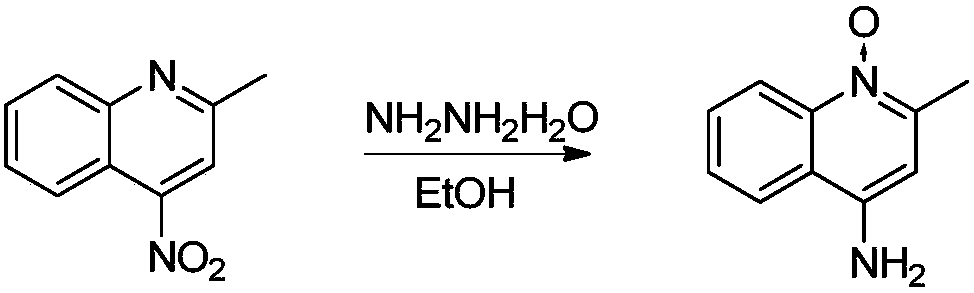Synthesis method of 4-aminoquinaldine
A synthesis method, quinaldine technology, applied in the field of synthesis of 4-aminoquinaldine, can solve the problems of high risk and inconvenient operation, and achieve the effect of simple raw materials, simple operation, and simple separation and purification method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthetic method of existing 4-aminoquinaldine:
[0051]
[0052] ①Put 45.5ml (0.5mol) of double-distilled aniline, 137.0ml (0.7mol) of ethyl acetoacetate, 196ml of benzene and 2.0ml (0.01mol) of acetic acid into a 500mL three-necked bottle, heat and reflux until the water separator is free. Water brought out. After recovery of benzene under normal pressure, the low boilers are recovered by distillation under reduced pressure, and the residual liquid is the crude product of ethyl β-aniline crotonate (I), which is a light brown-red liquid, about 100 mL.
[0053] ②In a 500ml three-necked round bottom flask equipped with a dropping funnel, a sealed stirrer and an air condenser, place 120ml of the pilot oil, stir and heat to reflux. At the same time, 100ml of the obtained liquid (I) was quickly added, continued to stir and reflux for about 9 minutes, and cooled to room temperature. Add an appropriate amount of petroleum ether (60-90°C), and leave it overnight to pr...
Embodiment 2
[0058] 1. Synthesis of 2-methylquinoline N-oxide
[0059]
[0060] 143.1 g (1 mol) of 2-methylquinoline was dissolved in 1000 ml of acetic acid, and 300 ml of hydrogen peroxide (30%) was added, then heated to 80° C. and stirred for 4-5 hours.
[0061] Track the progress of the reaction with a thin layer of silica gel, developer: ethyl acetate, until the reaction is complete. Evaporate the acetic acid under reduced pressure, dissolve the residue in water, add an equal volume of dichloromethane and stir, separate the dichloromethane layer, dry it with anhydrous sodium sulfate, concentrate and separate by column (pure ethyl acetate) to obtain 2-methylquinoline N-oxide, light yellow solid crude product 156g, yield 98%. Proceed directly to the next reaction.
[0062] 2. Synthesis of 2-methyl-4-nitroquinoline N-oxide
[0063]
[0064] Dissolve 76g (0.48mol) of 2-methyl-4-nitroquinoline N-oxide in 507ml of nitric acid, add 101ml of sulfuric acid (the solution is dark red and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com