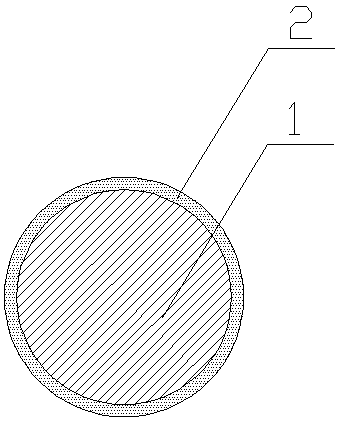
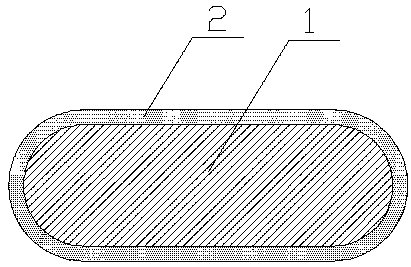
In-situ synthesized metal coating silver brazing rod and preparation method thereof
A technology of metal cladding and in-situ synthesis, applied in metal processing equipment, welding equipment, welding media, etc., can solve the problems of easy oxidation, difficulty in making rings, corrosion of solder cores, etc., and achieve high coating activity, Effect of improving wettability and fluidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] A preparation method for in-situ synthesis of metal-clad coating silver brazing rod, comprising the following steps:
[0028] a) Take Ag 34%, Cu 35.8%, Zn 27%, Sn 1.5%, P 0.4%, Ni 1%, Si0.15% and Li 0.15% according to the following mass percentages, and add each component into the crucible of the intermediate frequency smelting furnace , obtaining strip-shaped silver solder cores by smelting, casting, extruding and drawing;
[0029] b) Put the strip-shaped silver solder core into the ultrasonic cleaning equipment, add metal cleaning agent, and degrease and clean the surface of the strip-shaped silver solder core;
[0030] c) Prepare a pickling solution with sulfuric acid and water at a ratio of 1:10, put the strip-shaped silver solder core after decontamination into the pickling solution, and soak for 10 minutes at a temperature of 60°C;
[0031] d) Rinse the strip-shaped silver solder cores after pickling, then passivate them in a passivating agent, and then put the s...
Embodiment 2
[0035] A preparation method for in-situ synthesis of metal-clad coating silver brazing rod, comprising the following steps:
[0036] a) Take Ag 50%, Cu 32%, Zn 14%, Sn 1.75%, P 0.5%, Ni 1.5%, Si0.1% and Li 0.15% according to the following mass percentages, and add each component into the crucible of the intermediate frequency smelting furnace , obtaining strip-shaped silver solder cores by smelting, casting, extruding and drawing;
[0037] b) Put the strip-shaped silver solder core into the ultrasonic cleaning equipment, add metal cleaning agent, and degrease and clean the surface of the strip-shaped silver solder core;
[0038] c) Prepare a pickling solution with sulfuric acid and water at a ratio of 1:10, put the strip-shaped silver solder core after decontamination into the pickling solution, and soak for 20 minutes at a temperature of 40°C;
[0039] d) Rinse the strip-shaped silver solder core after pickling, then passivate it in a passivating agent, and then put the stri...
Embodiment 3
[0043] A preparation method for in-situ synthesis of metal-clad coating silver brazing rod, comprising the following steps:
[0044] a) Take Ag 40%, Cu 30%, Zn 25%, Sn 2.5%, P 0.5%, Ni 1.6%, Si 0.2% and Li 0.2% according to the following mass percentages, and add each component into the crucible of the intermediate frequency smelting furnace, Obtaining strip-shaped silver solder cores by smelting, casting, extruding and drawing;
[0045] b) Put the strip-shaped silver solder core into the ultrasonic cleaning equipment, add metal cleaning agent, and degrease and clean the surface of the strip-shaped silver solder core;
[0046] c) Prepare a pickling solution with sulfuric acid and water at a ratio of 1:10, put the strip-shaped silver solder core after decontamination into the pickling solution, and soak for 15 minutes at a temperature of 70°C;
[0047] d) Rinse the strip-shaped silver solder core after pickling, then passivate it in a passivating agent, and then put the strip-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com