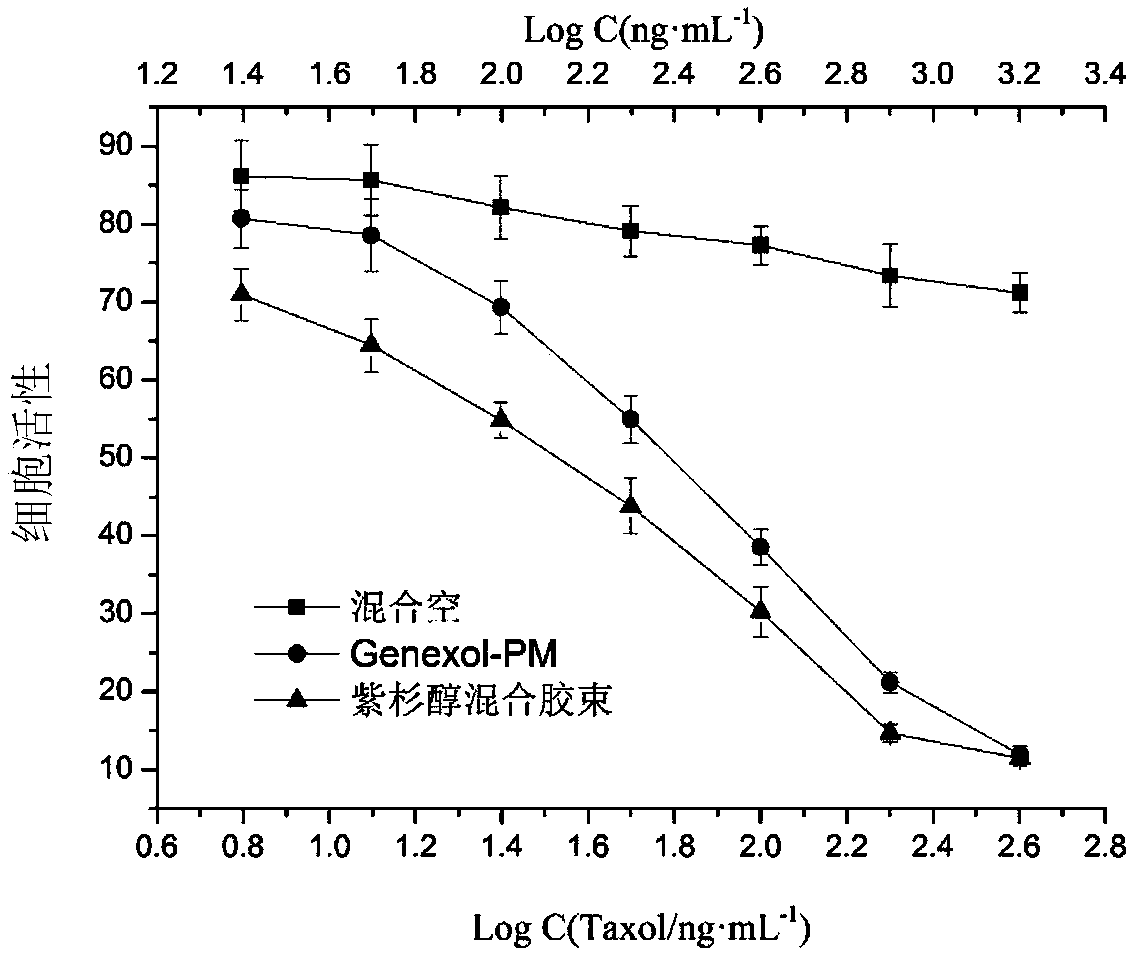
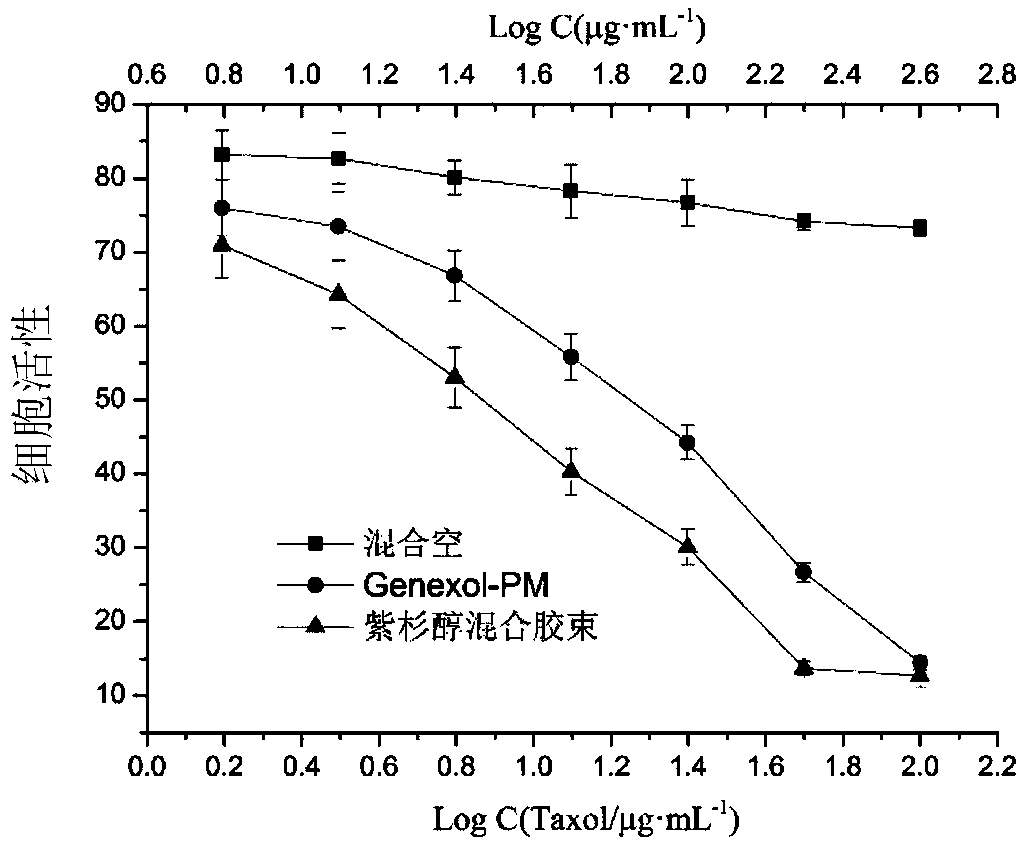
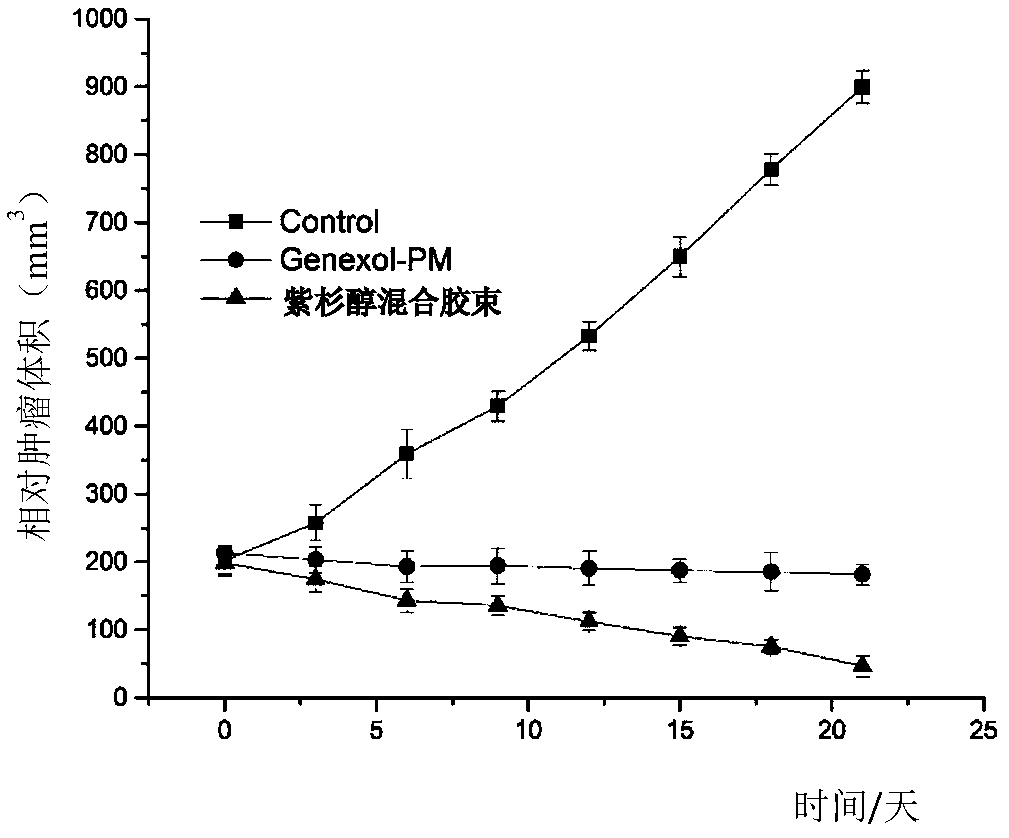
Blank mixed micelle, and preparation method and applications thereof
A mixed micelle and blank technology, which is applied to blank mixed micelles and their preparation and application fields, can solve the problems of membrane toxicity, large particle size, and non-single molecular structure components of membrane materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0206] Preparation of 2% erythrocyte suspension: Take blood from healthy rabbits, put it into a conical flask containing glass beads and shake it for 10 minutes, or stir the blood with a glass rod to remove fibrinogen and make it into defibrillated blood. Add about 10 times the amount of 0.9% sodium chloride solution, shake well, centrifuge at 1000-1500 rpm for 15 minutes, remove the supernatant, and wash the precipitated red blood cells with 0.9% sodium chloride solution for 2-3 times according to the above method. until the supernatant does not appear red. The resulting erythrocytes were made into a 2% suspension with 0.9% sodium chloride solution for testing.
[0207] Inspection method: Take 5 clean glass test tubes, No. 1 and No. 2 tubes are the test sample tubes, No. 3 tube is the negative control tube, No. 4 tube is the positive control tube, and No. 5 tube is the test product control tube. Add 2% erythrocyte suspension, 0.9% sodium chloride solution, and purified water...
preparation Embodiment 1
[0231] Preparation Example 1 Preparation of Ginsenoside Rg5H
[0232] Dissolve 10g of 20(R)-ginsenoside Rg3 in 20mL of pyridine, add 10mL of acetic anhydride dropwise in an ice-water bath, then add an appropriate amount (usually a catalytic amount, such as 1g) of catalyst DMAP, slowly warm up to room temperature, and react for 10 hours. TLC detected that the raw material point disappeared, concentrated under reduced pressure to remove the organic solvent, extracted 3 times with 200 mL / ethyl acetate, combined the organic phases, washed 3 times with 2M hydrochloric acid, washed 3 times with saturated sodium bicarbonate, dried over anhydrous sodium sulfate, and reduced pressure Concentration to dryness afforded Rg3 acetylated product.
[0233] Dissolve 10g of Rg3 acetylated product in 50mL of dichloromethane, add 1g of boron trifluoride diethyl ether and 1g of triethylsilane successively in an ice-water bath, slowly warm up to room temperature, continue the reaction at room tempe...
preparation Embodiment 2
[0239] Preparation Example 2 Preparation of Ginsenoside Rg5H1(E), Rg5H1(Z), Rk1H
[0240] Weigh 10g of the 20(R)-Rg3 acetylation product, dissolve it in 50mL of methanol, add 1g of palladium carbon, under normal temperature and pressure, pass in hydrogen, stir for 4-6 hours until the reaction liquid does not absorb hydrogen, TLC detects to the raw material point Disappeared, filtered off the palladium carbon, concentrated under reduced pressure to remove methanol, and dried to obtain 20(R)-Rg3 acetylated hydrogenation product.
[0241] Weigh 10g of 20(R)-Rg3 acetylated hydrogenation product, dissolve it in 50mL of toluene, add 10g of p-toluenesulfonic acid, slowly raise the temperature to 90°C and reflux, react for 4 hours, TLC detects that the raw material point disappears, cool, 100mL / Washed three times with subsaturated sodium bicarbonate solution, dried over anhydrous sodium sulfate, and concentrated to dryness under reduced pressure to obtain a mixture of crude acetylate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com