Glucagon-like peptide-1 analogue sustained release microsphere as well as preparation method thereof
A technology of glucagon and sustained-release microspheres, which is applied in the field of sustained-release preparations and preparations of glucagon-like peptide-1 analogues, and can solve the problem that microspheres cannot achieve the release effect, affect the therapeutic effect of the preparation, and cause blood Avoid problems such as increased drug concentration, achieve uniform size, reduce the number of administrations, and control blood sugar
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
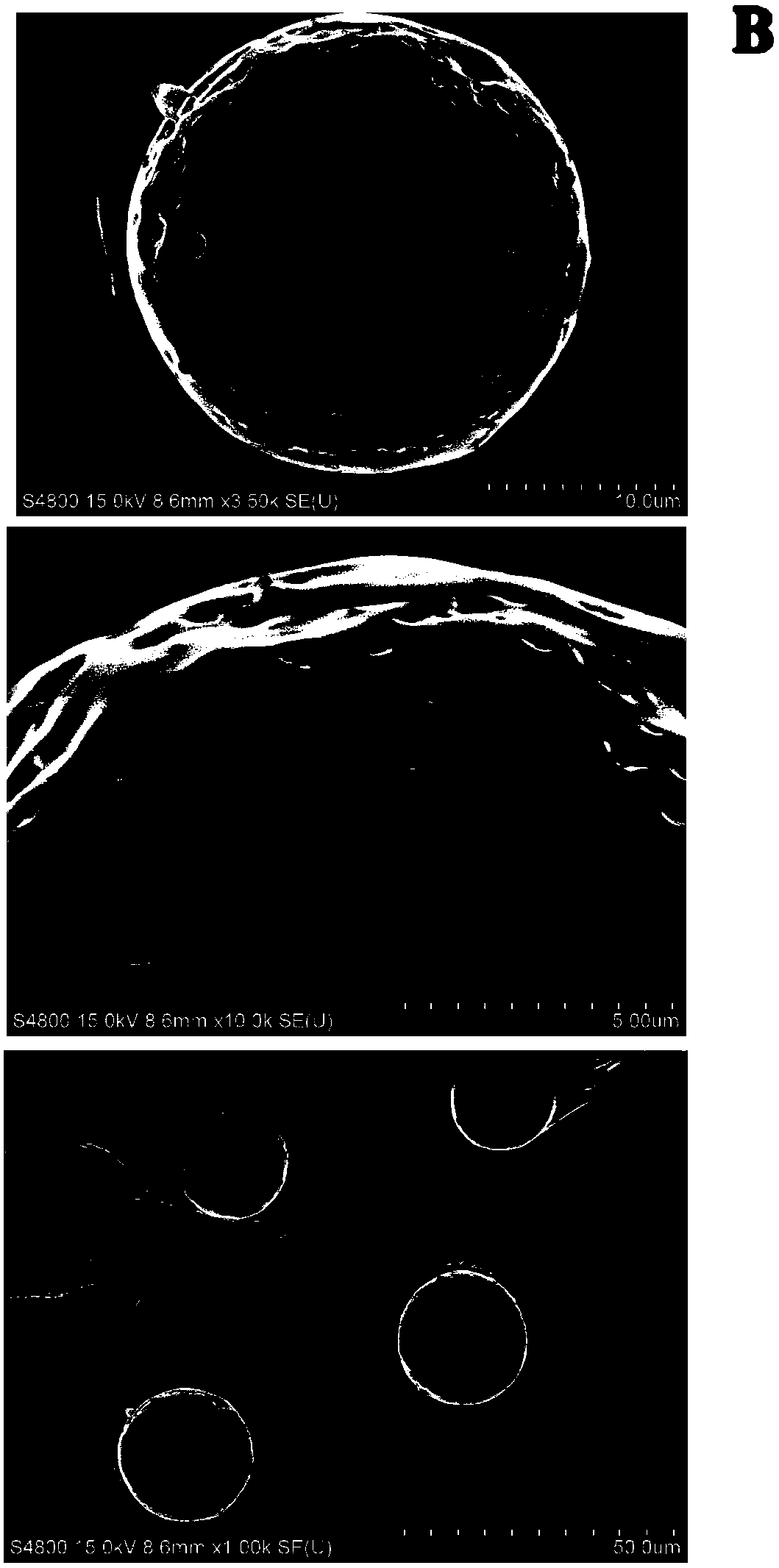
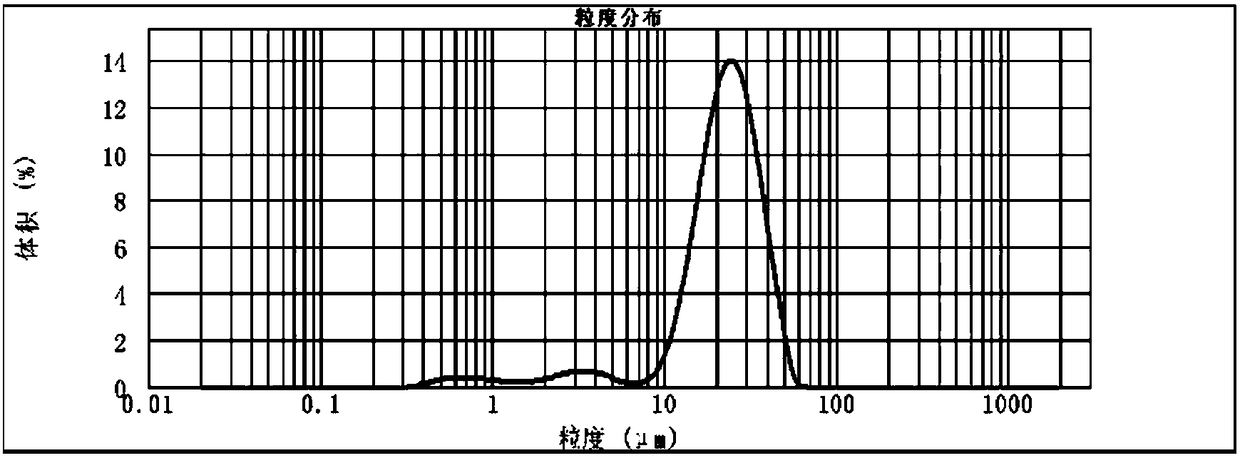
Image
Examples
Embodiment 1
[0076] Accurately weigh 6 mg of GLP-1 analogue and dissolve it in 200 μl of water to configure an inner aqueous phase solution with a concentration of 30 mg / ml, and add the inner aqueous phase to 1 ml of polylactic acid polyglycolic acid (LA:GA= 50:50Mw=17kDa) in dichloromethane solution (oil phase), the volume ratio of the inner water phase to the oil phase is 1:5. The inner water phase is mixed with the oil phase, and ultrasonicated with a power of 50 W for 3 minutes in an ice-water bath to obtain a W / O emulsion. Slowly inject the obtained colostrum into 20ml of the external water phase containing 2% polyvinyl alcohol and 2.5% sodium chloride with a 25G syringe, and use a homogenizer to emulsify at a speed of 3000rpm for 1 minute to form a W / O / W double emulsion . Add the above-mentioned double emulsion to 200ml of 0.1% polyvinyl alcohol aqueous solution, solidify for 4 hours, and after the organic solvent is completely volatilized, centrifuge the suspension at 3000rpm for f...
Embodiment 2
[0078] Sprinkle 4mg of polypeptide lyophilized powder (S) into ethyl acetate solution (O) containing 1ml of 100mg / ml lactic acid polyglycolic acid (LA:GA=50:50Mw=17kDa), mix and use in an ice-water bath Sonicate the probe at 50W for 3 minutes to obtain the S / O suspension. Slowly inject the S / O suspension into 20ml of the external aqueous phase containing 2% sodium lauryl sulfate and 2.5% sodium chloride with a 25G syringe, and emulsify with a homogenizer at 3000rpm for 1 minute to form S / O / W lotion. Pour the above emulsion into 200ml of aqueous solution containing 0.1% PVA. After curing for 4 hours, after the organic solvent has evaporated completely, centrifuge the suspension at 8000rpm for five minutes, wash it with water for five times, and set it at 0.12MPa, -40 Freeze-dry at ℃. The obtained microspheres have an average particle size of 3.68 μm and a drug loading of 1.5%.
Embodiment 3
[0080]Accurately weigh 6 mg of GLP-1 analogue and dissolve it in 200 μl of water containing 10% PEG as a protective agent to form an internal aqueous phase solution with a polypeptide concentration of 30 mg / ml, and add the internal aqueous phase to 1 ml of polylactic acid poly(lactic acid) with a concentration of 100 mg / ml. In the chloroform solution (oil phase) of glycolic acid (LA:GA=50:50Mw=17kDa), the ratio of the inner water phase to the oil phase is 1:5. The inner water phase is mixed with the oil phase and ultrasonicated for 3 minutes using a probe ultrasonic method with a power of 50W in an ice-water bath to obtain a W / O emulsion. Slowly inject the obtained colostrum into 20ml of the external aqueous phase containing 2.5% polysorbate and 2.5% sodium chloride with a 25G syringe, and use a homogenizer to emulsify at a speed of 3000rpm for one minute to form a W / O / W complex milk. Pour the above double emulsion into, for example, 200ml of 0.1% polyvinyl alcohol aqueous so...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com