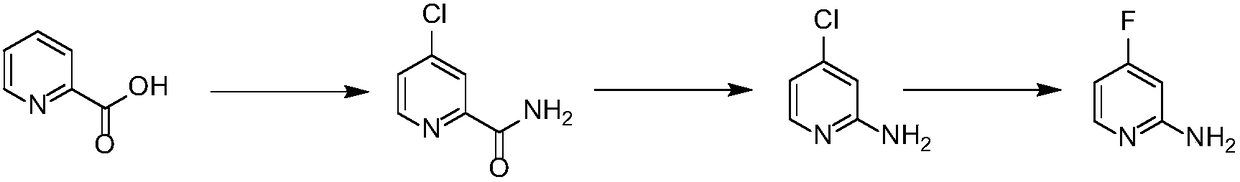
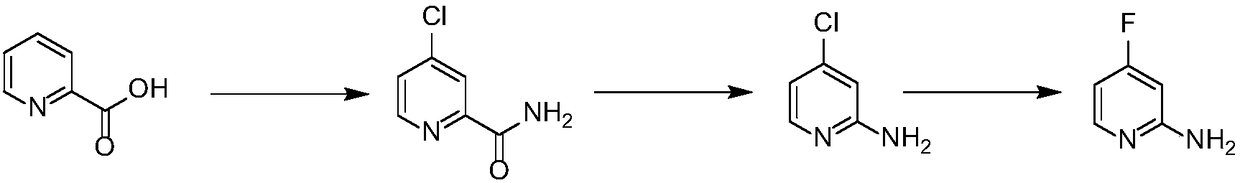
Preparation method of 2-amino-4-fluoropyridine
A technology of fluoropyridine and amino, which is applied in the field of preparation of 2-amino-4-fluoropyridine, can solve the problems of large amount of three wastes, high cost, expensive raw materials, etc., and achieve the effect of safe operation, simple operation and short steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Embodiment 1: Preparation of 2-amino 4-fluoropyridine
[0021] Slowly add 580 grams of 2-pyridinecarboxylic acid into 1.68 kilograms of thionyl chloride, add 48 grams of sodium bromide therein, and reflux the reaction solution for 2-16 hours until the reaction of the raw materials is complete, and the thionyl chloride is recovered. Obtain 680 grams of 4-chloropyridine-2-acyl chloride; 4-chloropyridine-2-acyl chloride is added in batches to 4 kg of 30% aqueous ammonia, stirred at room temperature for 5-16 hours until the reaction is complete, and most of the water is removed , the temperature was lowered to 0-5° C., and a white solid was precipitated and dried to obtain 4-chloropyridine-2-amide, 550 g, with a yield of 95%. 1 H NMR (300MHz, DMSO-d 6 ): δ8.60(d, J=5.7Hz, 1H), 8.16(bs, 1H), 8.01(d, J=2.1Hz, 1H), 7.78(bs, 1H), 7.74(d, J=2.1Hz , 1H).
[0022] Add 550 grams of 4-chloropyridine-2-amide in batches to 10 kg of sodium hypochlorite solution with a mass concentra...
Embodiment 2
[0024] Embodiment 2: the preparation of 2-amino 4-fluoropyridine
[0025] Slowly add 40 grams of 2-pyridinecarboxylic acid into 116 grams of thionyl chloride. After the addition is complete, add 1 gram of N,N-dimethylformamide to it, and reflux the reaction solution for 2-16 hours until the reaction of the raw materials is complete. Thionyl chloride was recovered to obtain 46 grams of 4-chloropyridine-2-acyl chloride; 4-chloropyridine-2-acyl chloride was dissolved in 200 ml of 1,4-dioxane, and ammonia gas was passed into it for 5-16 hours After the reaction was completed, most of the solvent was removed, the temperature was lowered to 0-5° C., and a white solid was precipitated and dried to obtain 4-chloropyridine-2-amide, 37 g, with a yield of 93%.
[0026] Add 35 grams of 4-chloropyridine-2-amide in batches to the calcium hypochlorite aqueous solution, which is prepared from 78 grams of calcium hypochlorite and 1 liter of water, and heat up to 60-80°C for reaction after addi...
Embodiment 3
[0028] Embodiment 3: Preparation of 2-amino 4-fluoropyridine
[0029] Slowly add 160 grams of 2-pyridinecarboxylic acid into 460 grams of thionyl chloride. After the addition, 35 grams of sodium chloride is added thereto, and the reaction solution is refluxed for 2-16 hours until the reaction of the raw materials is complete, and the thionyl chloride is recovered. Obtain 170 grams of 4-chloropyridine-2-acyl chloride; 4-chloropyridine-2-acyl chloride was dissolved in 2000 milliliters of tetrahydrofuran, and ammonia gas was passed into it for 5-16 hours until the reaction was complete, most of the solvent was removed, and the temperature was lowered to 0 -5°C, a white solid was precipitated and dried to obtain 4-chloropyridine-2-amide, 132 g, yield 90%.
[0030] Slowly add 236 grams of bromine dropwise into aqueous sodium hydroxide solution prepared by dissolving 118 grams of sodium hydroxide in 1 liter of water, and add 120 grams of 4-chloropyridine-2-amide to the reaction solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com