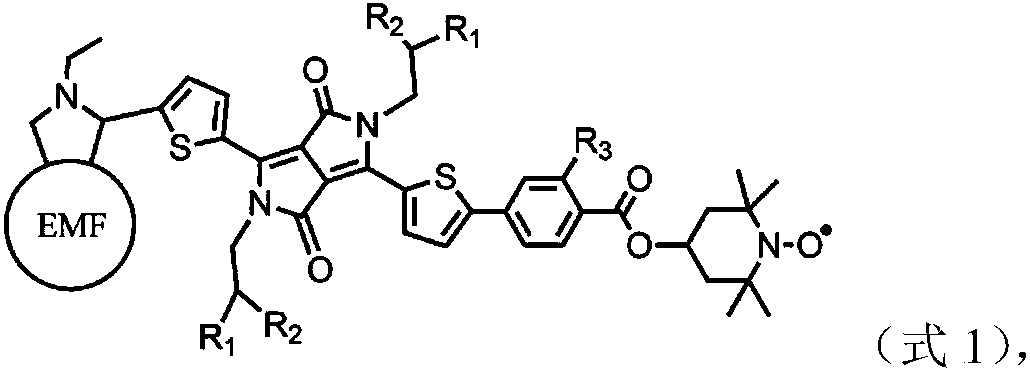
Metallofullerene nitroxide free radical derivative as well as preparation method and application thereof
A technology of fullerene nitroxide radical and metallofullerene, which can be used in the preparation of carbon-based compounds, the preparation of organic compounds, chemical instruments and methods, etc., and can solve the problems of complex catalyst components, poor selectivity, and peroxidation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] Example 1 metal fullerene Sc 3 N@C 80 Preparation method of nitroxide free radical derivative
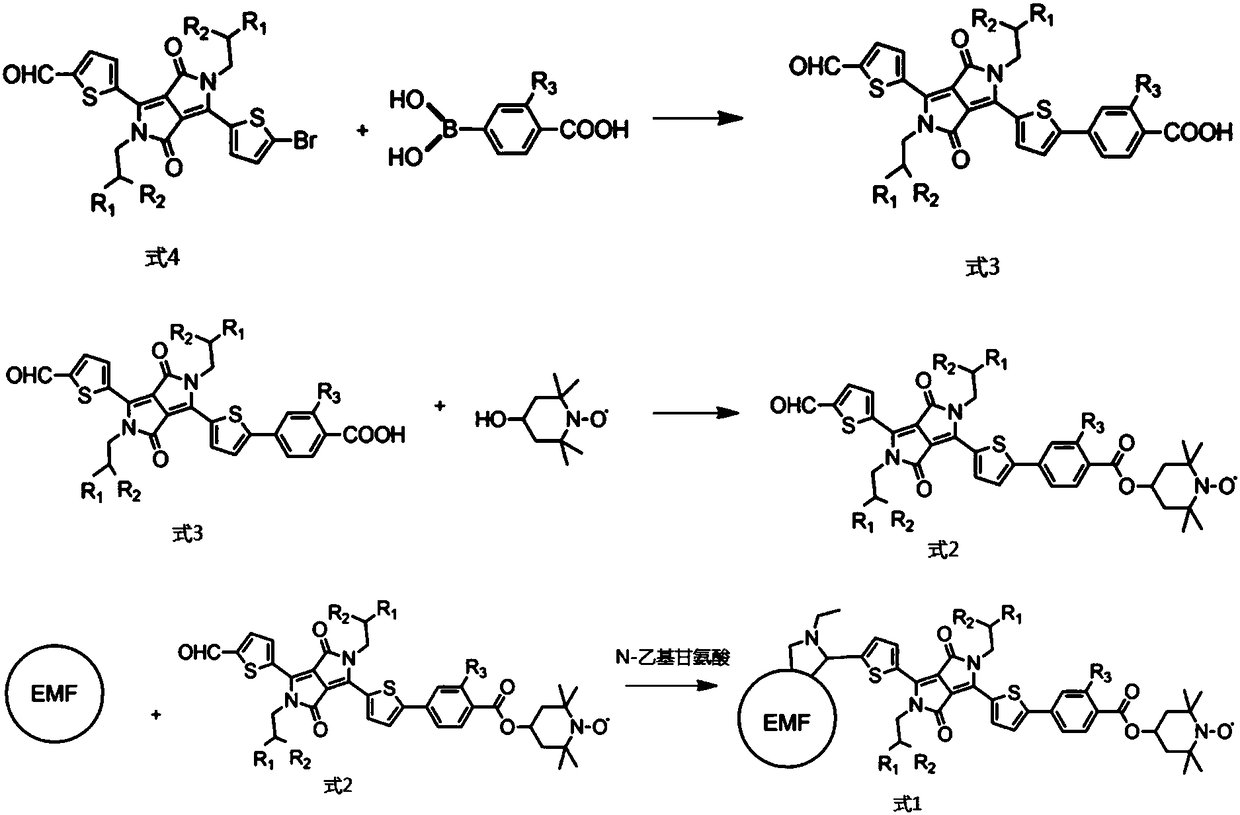
[0056] (1) The preparation method of DPP-carboxyl group,
[0057]
[0058] Including: take 50mg (ie 0.0672mmol) of DDP (ie R in formula 4 1 for-C 4 h 9 , the R 2 for-C 6 h 13 ), 22.3mg (ie 0.1344mmol) of 4-carboxyphenylboronic acid (ie R 3 For -H), 3.88mg (that is, 0.00336mmol) of 4-(triphenylphosphine) palladium as a catalyst, and 12.8mg (that is, 0.121mmol) of sodium carbonate that provide an alkaline environment are dissolved in 20ml of 1,4-diox In the mixture of six rings, 20ml of dichloromethane and 4ml of water, under magnetic stirring, use a double-row tube to degas three times, then raise the temperature to 110°C under an inert atmosphere of argon, and react overnight; the reaction is complete Finally, wash with 50ml of 0.1M hydrochloric acid aqueous solution, then wash with deionized water, and finally extract three times with 30ml of dichloromethane, com...
Embodiment 2
[0076] Metallofullerene Sc 3 N@C 80 The reaction of catalyzing the conversion of enol compounds by derivatives of nitroxide radicals is as follows:
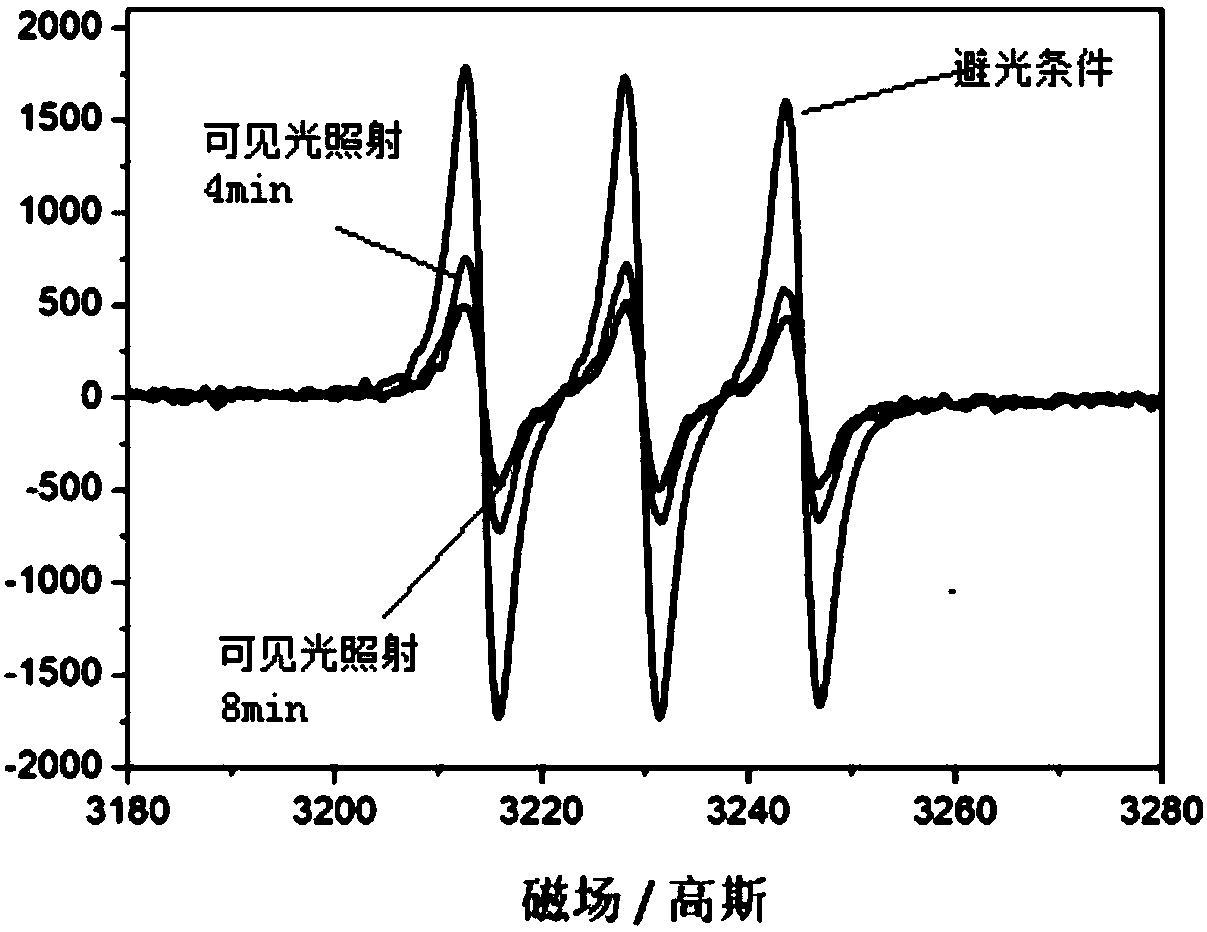
[0077] Metal fullerene Sc obtained in embodiment 1 3 N@C 80 Nitroxide radical derivatives are mixed with different small molecule enol compounds (see the next paragraph for specific reaction conditions), irradiated under visible light conditions, stirred for a period of time, and detected by gas chromatography. The results show that for different kinds of enols, metallofullerene Sc 3 N@C 80 When the nitrogen oxide free radical derivative is used as the catalyst, the selectivity is very good, the double bonds of the olefins are not destroyed, and the conversion of alcohols to aldehydes has occurred.
[0078] Reaction conditions: 0.5mmol enol compound substrate, 5×10 -3 mmolerene metal Sc 3 N@C 80 Nitroxide radical derivatives are used as catalysts, 0.5mmol of n-octane is added as an internal standard, dissolved in 500μL of...
Embodiment 3
[0084] Metallofullerene Sc 3 N@C 80 The reaction of the conversion of polyhydric alcohols that derivatives of nitroxide radicals participate in is as follows:
[0085] As shown in the following reaction formula, for reaction (1), add metal fullerene Sc obtained in Example 1 3 N@C 80Nitroxide free radical derivatives react with the corresponding substrate, 1,5-hexanediol to generate 87% of 5-hydroxyhexanal and 12% of 6-hydroxyhexyl-2-one. The reaction system is as follows: 0.5mmol 1,5-Hexanediol, 5 x 10 -3 mmolerene metal Sc 3 N@C 80 Nitroxide radical derivatives were used as catalysts, 0.5 mmol of n-octane was added as an internal standard, dissolved in 500 μL of toluene, mixed thoroughly and exposed to visible light for 5 hours, and the obtained products were detected by gas chromatography.
[0086] For reaction (2) (1-(4-(hydroxymethyl) phenyl) ethanol reacts and generates about 78% 4-(1-hydroxyethyl-benzaldehyde), less than 2% of 1-(4-( Hydroxymethyl) phenyl) ethyl k...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com