Method for recycling valuable metal in waste nickel-cobalt-manganese ternary lithium-ion battery
A lithium-ion battery and valuable metal technology, which is applied in the field of waste lithium-ion battery recycling, can solve the problems of high impurity removal depth requirements of leaching solution, long co-precipitation reaction time, and high recovery cost, so as to improve the recovery effect and improve the comprehensive recovery rate. , the effect of high recovery rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
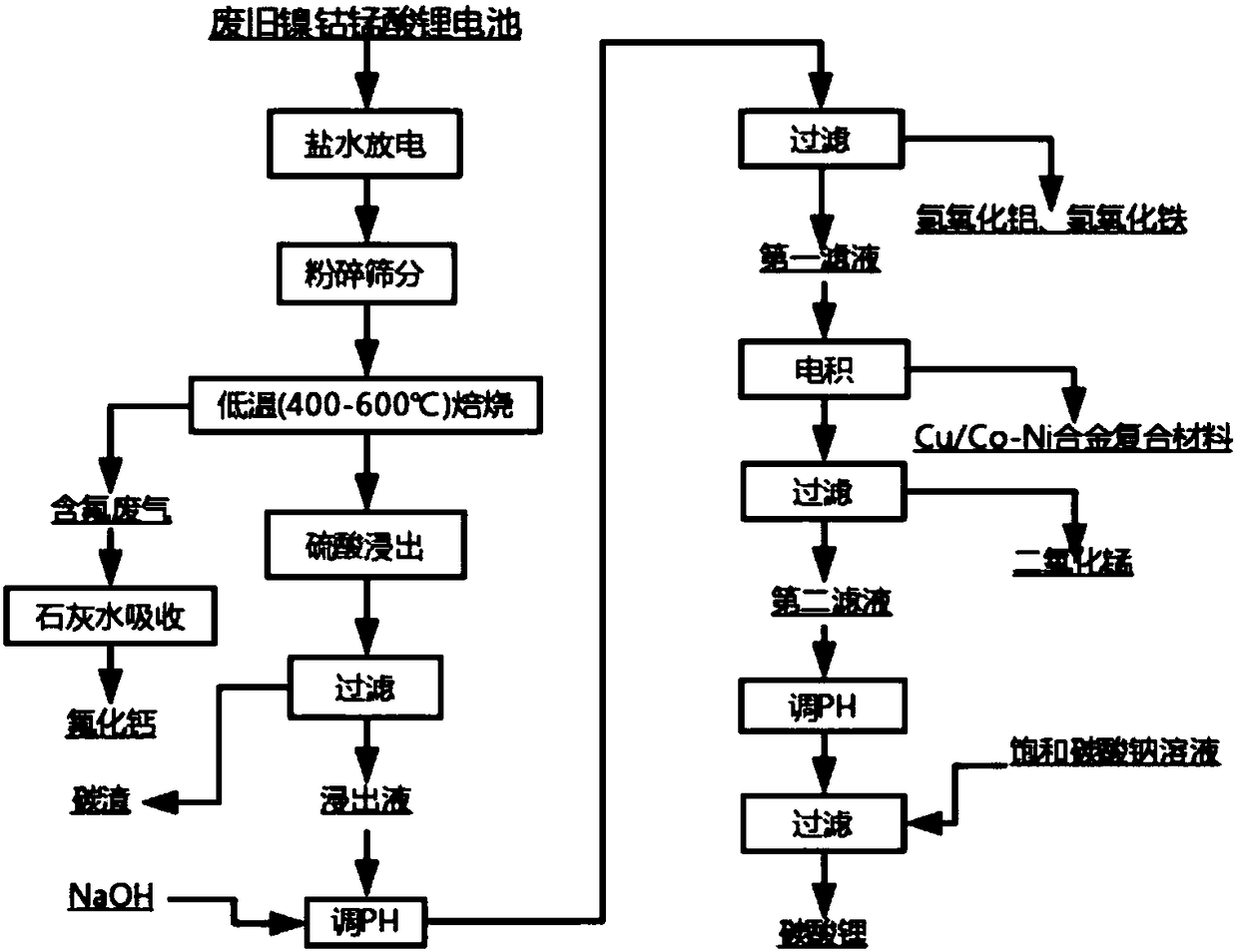
[0053] Soak the waste nickel-cobalt-lithium-manganese-oxide battery in 5% sodium chloride solution until the end-of-discharge voltage is 1V, mechanically crush and sieve the broken materials with a particle size of less than 0.1mm and send them to the roasting process. The crushed material is roasted in air at 450°C for 1 hour to remove the binder, and the exhaust gas from roasting is absorbed with 50mg / L lime aqueous solution. After roasting, the material is sent to the leaching process. The leaching parameters are as follows: 1mol / L H 2 SO 4 , 2g / L sucrose, solid-to-liquid ratio 50g / L, leaching time 60min, leaching temperature 60°C, filter and separate to obtain carbon residue and leaching solution. Then add an appropriate amount of 1mol / L NaOH solution to the leaching solution, adjust the pH to 3-5 and filter to obtain Fe(OH) 3 and Al(OH) 3 . Then the filtrate (removal filtrate; wherein, Li + 、Ni 2+ 、Co 2+ , Mn 2+ The ion concentrations are 1.71g / L, 5.54g / L, 3.32g / L,...
Embodiment 2
[0056] Soak the waste nickel-cobalt-lithium-manganese-oxide battery in 10% sodium chloride solution until the end-of-discharge voltage is 0.7V, mechanically crush and sieve the fragments with a particle size of less than 0.2mm and send them to the roasting process. The crushed material is roasted in air at 500°C for 2 hours to remove the binder, and the exhaust gas from roasting is absorbed with 60 mg / L lime aqueous solution. After roasting, the material is sent to the leaching process. The leaching parameters are as follows: 2mol / L H 2 SO 4 , 4g / L sucrose, solid-to-liquid ratio 70g / L, leaching time 90min, leaching temperature 70°C, filter and separate to obtain carbon residue and leaching solution. Then add an appropriate amount of 2mol / L NaOH solution to the leaching solution, adjust the pH to 4-5 and filter to obtain Fe(OH) 3 and Al(OH) 3 . Then the filtrate (removal filtrate; wherein, Li + 、Ni 2+ 、Co 2+ , Mn 2+ The ion concentrations of the ions are 2.39g / L, 7.86g / L...
Embodiment 3
[0059] Soak the waste nickel-cobalt-lithium-manganese-oxide battery in 15% sodium chloride solution until the end-of-discharge voltage is 0.5V, mechanically crush and sieve the fragments with a particle size of less than 0.25mm and send them to the roasting process. The crushed material is roasted in the air at 550°C for 3 hours to remove the binder, and the roasting waste gas is absorbed with 70mg / L lime aqueous solution. After roasting, the material is sent to the leaching process. The leaching parameters are as follows: 2.5mol / L H 2 SO 4 , 6g / L sucrose, solid-to-liquid ratio 90g / L, leaching time 120min, leaching temperature 80°C, filter and separate to obtain carbon residue and leaching solution. Then add an appropriate amount of 3mol / L NaOH solution to the leaching solution, adjust the pH to 6 and filter to obtain Fe(OH) 3 and Al(OH) 3 . Then the filtrate (removal of impurities; wherein, Li + 、Ni 2+ 、Co 2+ , Mn 2+ The ion concentration of the ions are 3.12g / L, 9.26g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com