Near-infrared chlorinated aza-boron fluoride dye, and preparation method and application of dye
A technology of heterofluoroborane and nitrogen chloride is applied in the field of organic optoelectronic materials to achieve the effects of weakening the influence of detection signals, simple preparation, separation and purification processes, and abundant raw material sources.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: the synthesis of chlorine atom substituted azafluoroborane, the concrete synthetic route is as follows:
[0033]
[0034] Synthesis of Compound 1
[0035] Take a clean two-necked bottle, add magnetron, 3.40g of p-hydroxyacetophenone (about 25mmol), 24.14g of bromooctane (about 125mmol), 17.25g of potassium carbonate and 20mL of anhydrous N,N-dimethyl formamide solution. Under magnetic stirring, the reaction was carried out at 80°C for 24 hours. After the reaction, the mixture was extracted with water / dichloromethane for several times, and the organic phases were combined. Chromatographic column separation gave a colorless liquid (99% yield).
[0036] Synthesis of Compound 2
[0037] Take a clean two-necked bottle, add magnetron, 3.05g of p-hydroxybenzaldehyde (about 25mmol), 24.14g of bromooctane (about 125mmol), 17.25g of potassium carbonate and 20mL of anhydrous N,N-dimethylformamide solution. Under magnetic stirring, the reaction was carried ou...
Embodiment 2
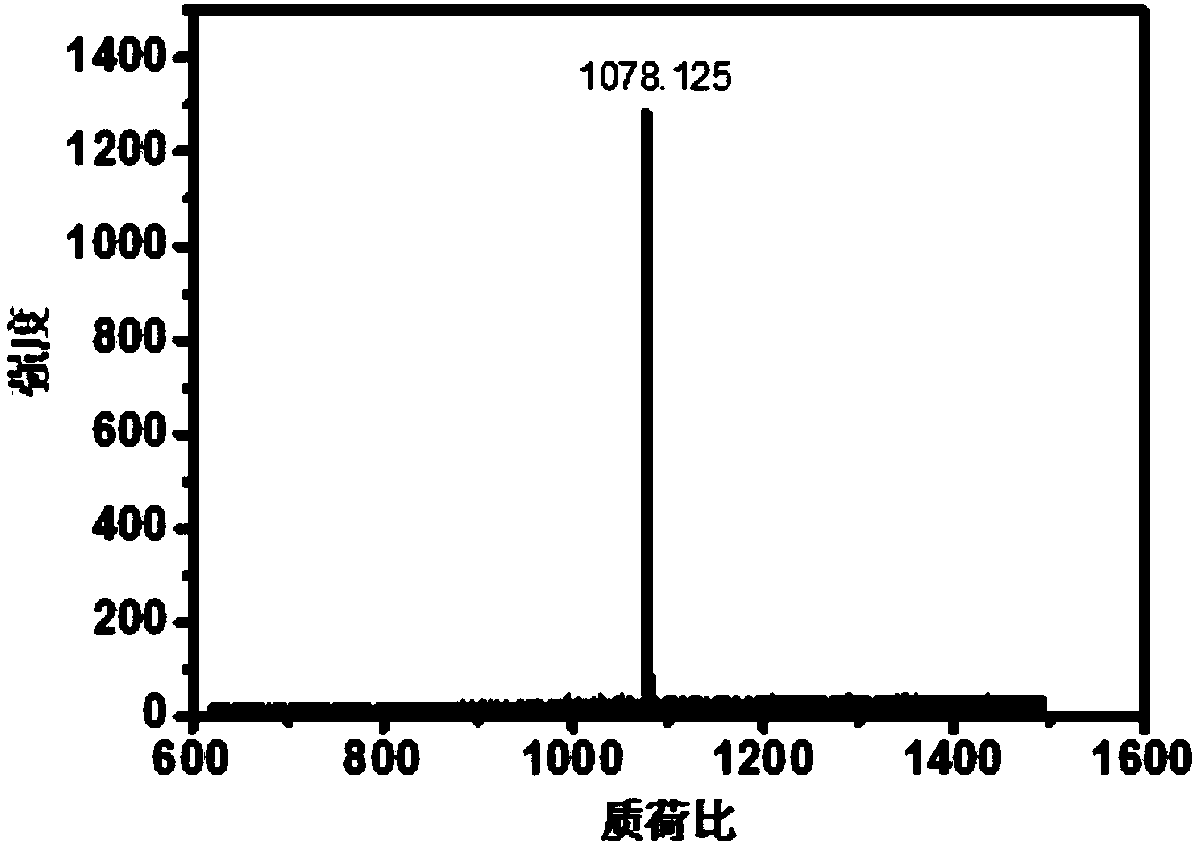
[0052] Example 2: Measurement of the molecular weight of compound 7
[0053] Take a small amount of compound 6, mix it with the matrix, and then spot the sample and measure it with MALDI-TOF. The results are as follows figure 1 , which preliminarily proved the correctness of compound 7 molecule.
[0054] [m / e](M, MALDI-TOF) theoretical value: 1077.59, experimental value: 1078.12.
Embodiment 3
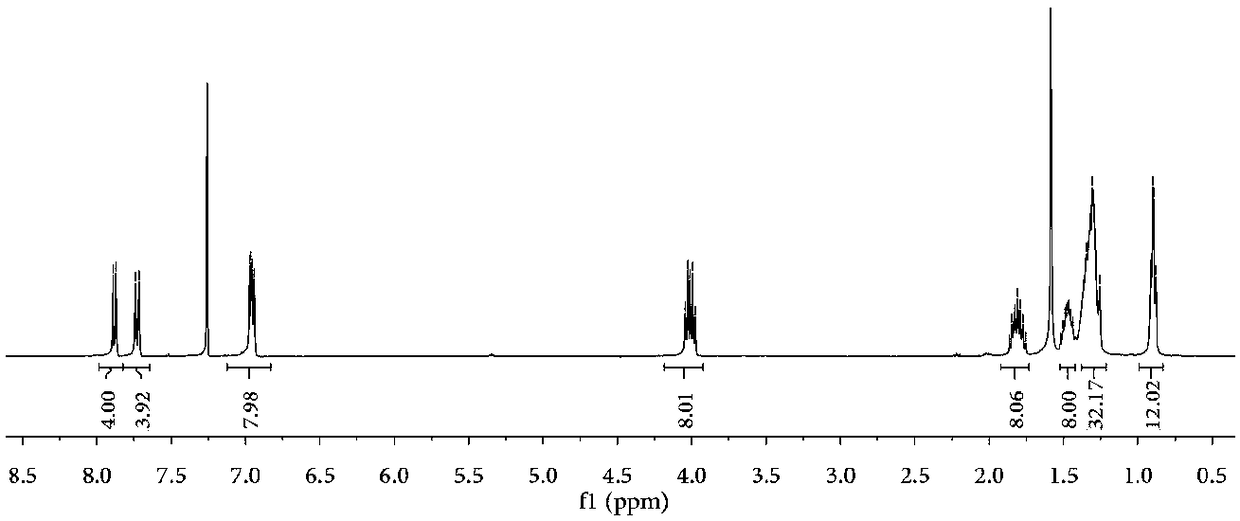
[0055] Example 3: NMR test of compound 7
[0056] Dissolve 0.5 mg of compound 7 in 0.5 mL of deuterated chloroform, and through nuclear magnetic test, the results are as follows figure 2 , which further proved the correctness of compound 7 molecule.
[0057] 1 H NMR (400MHz, CDCl 3 )d(ppm): δ7.97–7.90(m,4H),7.81–7.75(m,4H),7.01–6.93(m,8H),4.05–3.99(m,8H),1.87–1.76(m, 8H), 1.53–1.42 (m, 8H), 1.40–1.25 (m, 32H), 0.92–0.88 (m, 12H).
[0058] 13 C NMR (100MHz, CDCl 3 )d(ppm): δ161.32, 160.39, 155.60, 143.21, 138.92, 132.35, 122.83, 120.96, 114.16, 68.11, 31.85, 29.55, 29.10, 26.08, 22.69.
[0059] 19 F NMR (376.5MHz, CDCl 3 ):d(ppm):δ-131.51(q,2F).MALDI-TOF-MS(m / z):calcdfor C 64 H 84 BCl 2 F 2 N 3 O 4 , 1079.10; found, 1077.47.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com