Coumarin skeleton based Wittig reagent as well as preparation method and application thereof
A coumarin skeleton and reagent technology, applied in the field of biochemistry, can solve the problems of relying on large analytical instruments, complex and cumbersome sample pretreatment process, etc., and achieve the effects of excellent selectivity and sensitivity, low overall cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0034] The preparation method of the Wittig reagent based on coumarin skeleton comprises the following steps:
[0035] a. Dissolve R-substituted salicylaldehyde and ethyl acetoacetate in an organic solvent, add piperidine, and reflux for 4 to 12 hours to prepare intermediate 1; the molar weight of the ethyl acetoacetate is R-substituted salicyl 1.5 to 2.5 times that of aldehyde, and the molar weight of piperidine is 0.1 to 1.6 times that of R-substituted salicylaldehyde; the organic solvent is any one of absolute ethanol, isopropanol, acetonitrile or dichloromethane.
[0036] b. Dissolve intermediate 1 in glacial acetic acid, add liquid bromine and hydrobromic acid, and stir overnight at room temperature to prepare intermediate 2; the molar weight of the liquid bromine is 1.5 to 2.0 times that of intermediate 1, and the hydrogen The molar amount of bromic acid is 3 to 5 times that of intermediate 1.
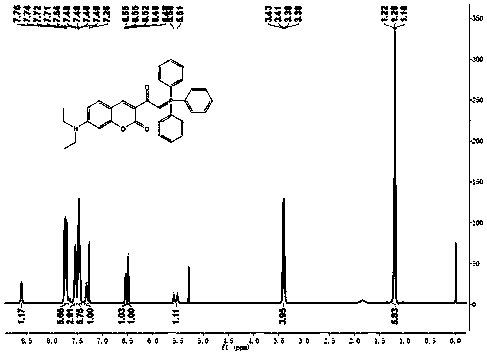
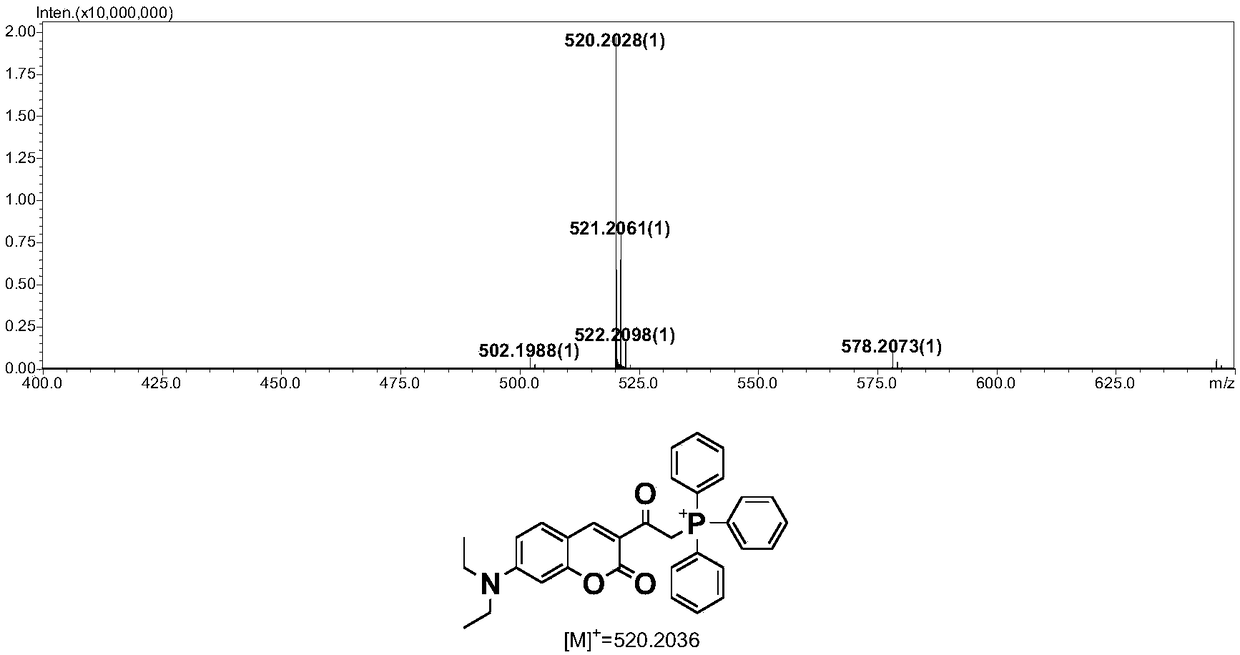
[0037] c. Intermediate 2, Ph 3 P (triphenylphosphine), KI (potassium iodid...
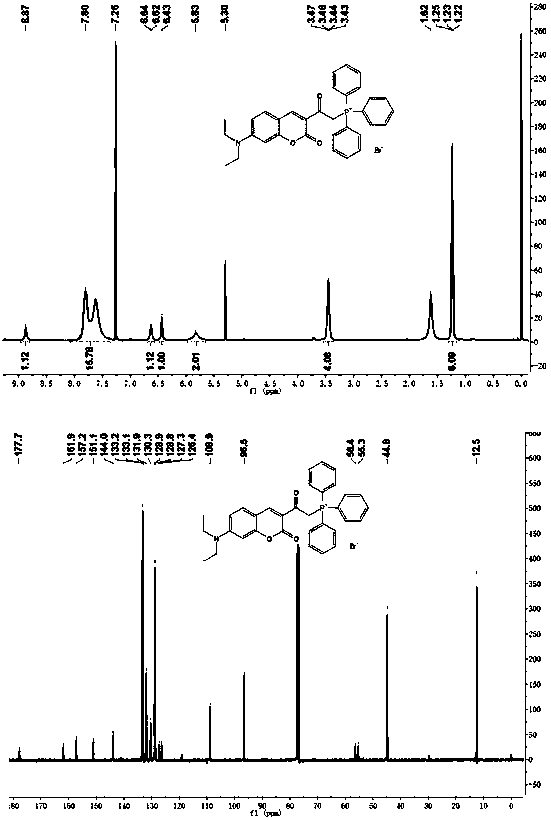
Embodiment 1
[0044] Example 1 Synthesis of 3-acetyl-7-(diethylamino)coumarin (compound 1):
[0045]
[0046] 4-(Diethylamino)salicylaldehyde (1.93g, 10mmol), ethyl acetoacetate (1.85g, 15mmol), piperidine (1mL, 39.5mmol) were dissolved in 200mL absolute ethanol, and refluxed at 80°C. After the reaction was complete, it was cooled to room temperature, filtered with suction, washed with ice ethanol, and dried in vacuo to obtain 2.15 g (8.3 mmol) of a shiny yellow solid with a yield of 83%.
[0047] 1 H NMR (400MHz, CDCl 3 )δ8.43(s,1H),7.39(d,J=9.0Hz,1H),6.61(dd,J=9.0,2.4Hz,1H),6.46(d,J=2.3Hz,1H),3.45( q,J=7.1Hz, 4H), 2.67(s,3H), 1.24(t,J=7.1Hz,6H).
Embodiment 2
[0048] Example 2 Synthesis of 3-(2-bromoacetyl)-7-(diethylamino)coumarin (compound 2):
[0049]
[0050] Compound 1 (1.5g, 5.78mmol), 50% hydrobromic acid aqueous solution (2.8g, 17.3mmol) were dissolved in 150mL glacial acetic acid, and liquid bromine (1.48g, 9.25mmol) was added dropwise under stirring conditions, and the dropwise , stirred overnight at room temperature. The solvent was removed under reduced pressure at 70°C, 100ml of water was added, the pH was adjusted to 8-9 with saturated sodium bicarbonate solution, extracted three times with dichloromethane, the organic phases were combined, dried over anhydrous sodium sulfate, filtered, and spin-dried. Chloroform was recrystallized to obtain 1.29 g (3.81 mmol) of an orange solid with a yield of 66%.
[0051] 1 H NMR (400MHz, CDCl 3 )δ8.53(s,1H),7.43(d,J=9.0Hz,1H),6.64(dd,J=9.0,2.5Hz,1H),6.48(d,J=2.4Hz,1H),4.77( s, 2H), 3.48 (q, J = 7.2Hz, 4H), 1.25 (t, J = 7.1Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com