Vinyl sulfonamide connector and application thereof
A technology of vinylsulfonamide and amide, applied in the field of selective modification of cysteine, can solve problems such as poor stability, and achieve the effect of good stability and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
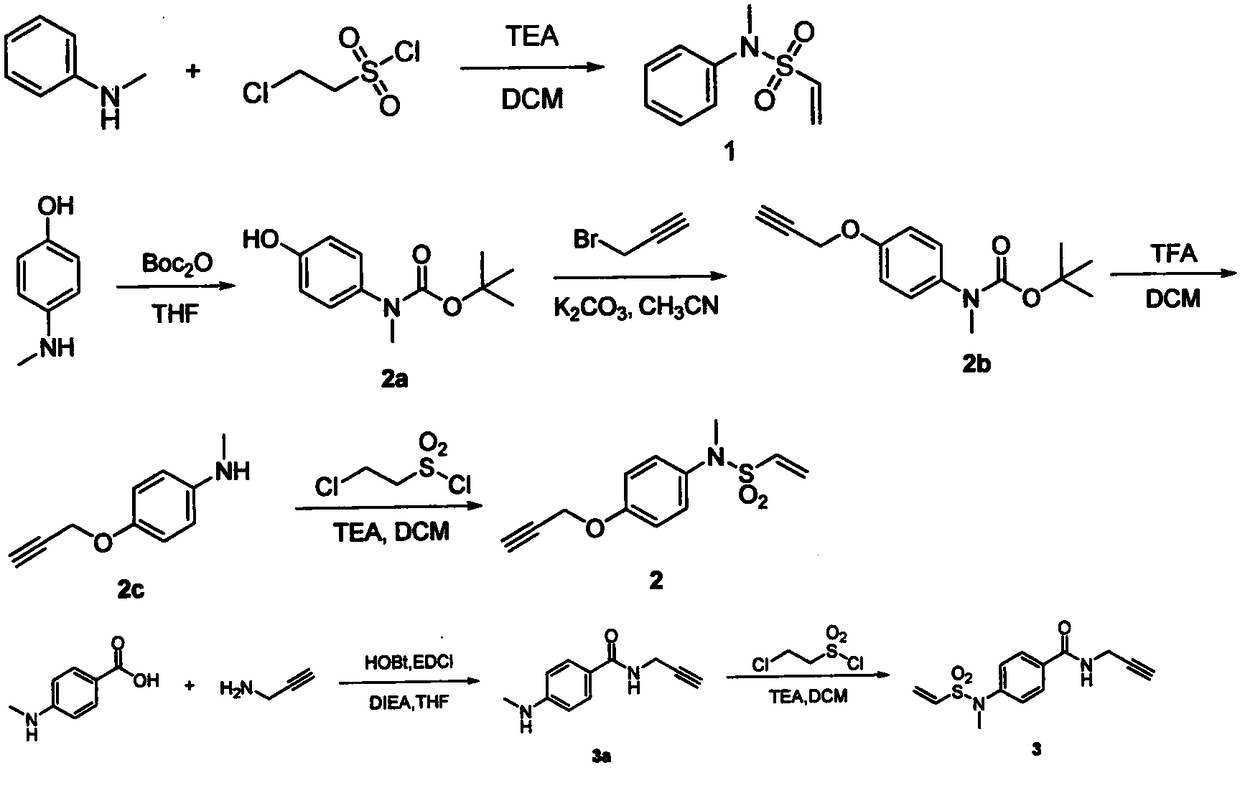
[0072] Preparation of vinylsulfonamide linkers 1-3 as figure 1 Shown:
[0073] Compound 1
[0074]
[0075] Dissolve N-methylaniline (0.536g, 5mmol) in dichloromethane (30mL), add triethylamine (1.518g, 15mmol) under ice-cooling, and slowly add 2-chloroethanesulfonyl chloride dropwise after fully cooling (0.978g, 6mmol), react in ice bath for 1h. After diluting the reaction solution with 100mL water, extract with dichloromethane (3*20mL), combine the organic phases, dry over anhydrous sodium sulfate, remove the solvent by rotary evaporation under reduced pressure to obtain a crude product, add 3mL CH 2 Cl 2 and 1.5g 60-100 mesh silica gel, mix well and spin dry. The crude product was subjected to silica gel column chromatography (petroleum ether: ethyl acetate = 5:1) to obtain white solid 1 (0.855 g, 4.34 mmol, 86.8%). 1 H NMR (500MHz, Chloroform-d) δ7.43-7.25(m, 5H), 6.47(dd, J=16.6, 9.9Hz, 1H), 6.21(d, J=16.6Hz, 1H), 6.03(d, J=10.0Hz, 1H), 3.26(s, 3H). 13 C NMR (12...
Embodiment 2
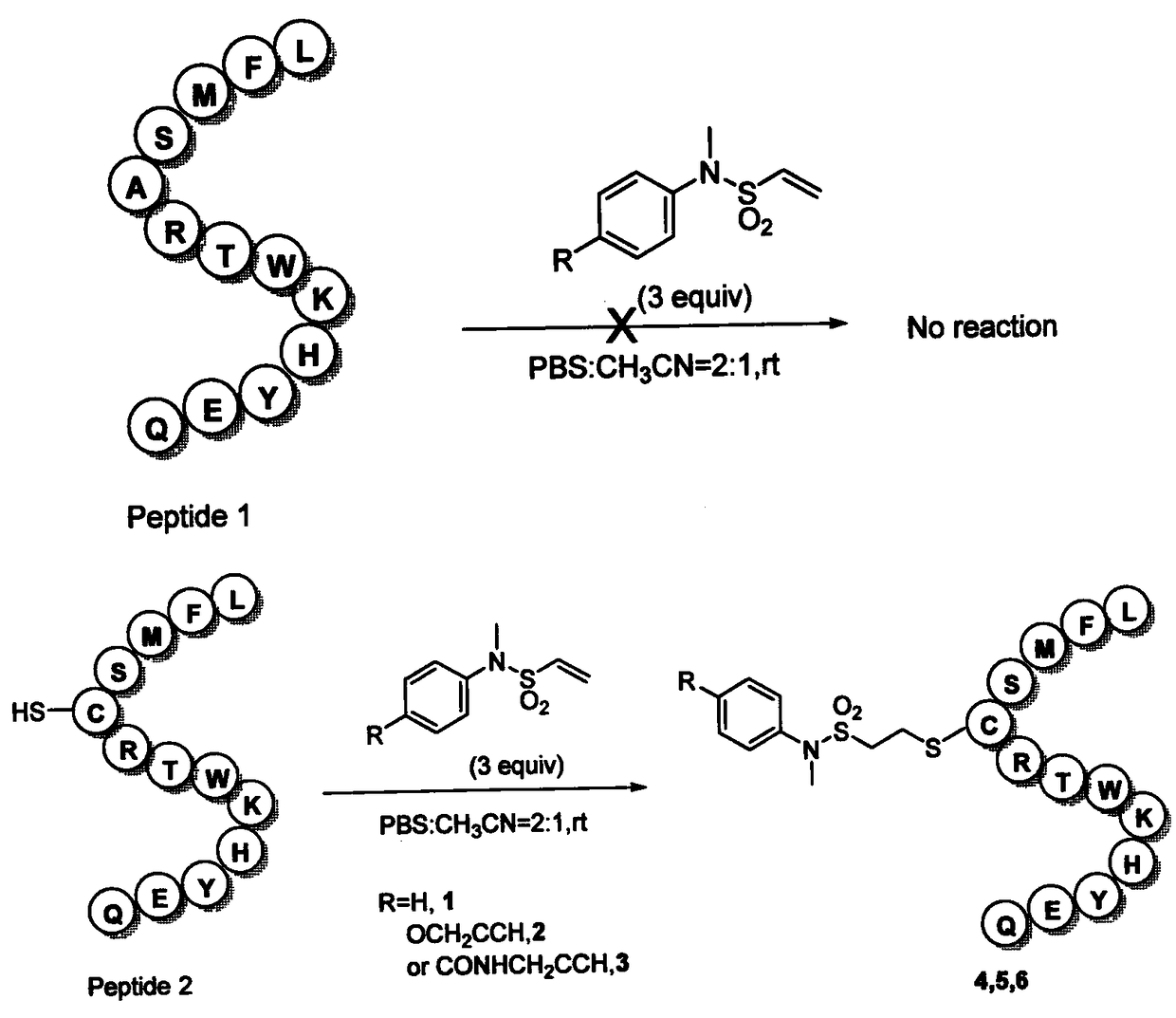
[0095] Selective modification of vinylsulfonamide linkers 1, 2, 3 with the polypeptide LFMSCRTWKHYEQ, such as figure 2 Shown:
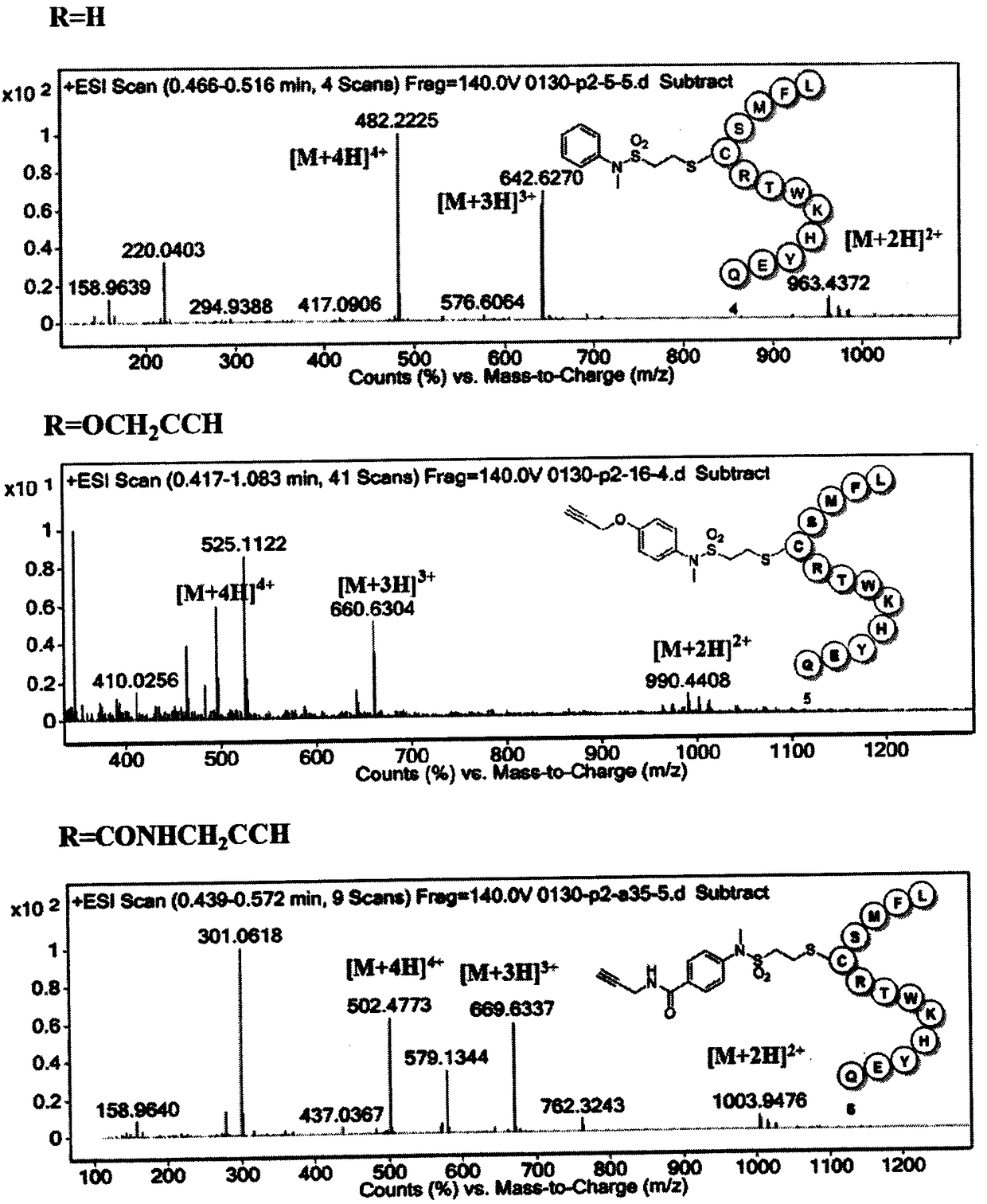
[0096] The polypeptide LFMSCRTWKHYEQ (Gill Biochemical) was dissolved in PBS (pH=7.4) buffer solution (product number Cat.NO.SH30256.0) to prepare a 1 mM solution, and the vinylsulfonamide linker was dissolved in acetonitrile to prepare a 6 mM solution. Add 200uL of polypeptide LFMSCRTWKHYEQ solution to three different wells of a microwell plate (330uL LABTIDE 96Round Well), then add 100uL of 3 different ethylenesulfonamide linker solutions, and react at room temperature on a microplate shaker for 2 Hours later, use HPLC (model Waters 1525 and SHIMADZU LC-30AD, the stationary phase is a C-18 silica gel column, and the mobile phase is CH 3 CN / H 2 O=10~100%, 0~10 minutes) analysis, judge product peak with HRMS-ESI, as image 3 shown.
[0097] The polypeptide LFMSARTWKHYEQ (Gill Biochemical) was dissolved in PBS (pH=7.4) buffer solution (product num...
Embodiment 3
[0099] like Figure 4 As shown, the conjugate of vinylsulfonamide linker 2 and polypeptide LFMSCRTWKHYEQ is further coupled with fluorescent substance fluorescein 7, coumarin 9; drug molecule camptothecin 8 and hydrophilic substance PEG 10:
[0100] Dissolve the polypeptide LFMSCRTWKHYEQ (Gill Biochemical) in PBS (pH=7.4) buffer solution (product number Cat.NO.SH30256.0) to make a 1 mM solution, and dissolve the vinylsulfonamide linker 2 in CH 3 CN is made into a 6mM solution, the azide reagent fluorescent substance fluorescein 7 and coumarin 9, the drug molecule camptothecin 8 and the hydrophilic substance PEG polymer 10 (molecular weight 2000) are dissolved in DMSO to make a 10mM solution, L -Sodium ascorbate dissolved in H 2 200mM solution in O, copper sulfate CuSO 4 Soluble in H 2 O to make a 100mM solution, and TBTA was dissolved in a 1:4DMSO-tert-butanol solution to make a 10mM solution.
[0101] Add 100uL of polypeptide LFMSCRTWKHYEQ solution and 50uL of ethylenesul...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com