Preparation method of tungsten/copper layer composite material based on surface nanocrystallization of tungsten sheet
A composite material and nano-technology, which is applied in the field of the preparation of mutually insoluble metal tungsten/copper layered composite materials, can solve the problems of destroying the consistency of the system components, unable to use large-scale, difficult to control the coating, etc., and achieve the production cost. Inexpensive, easy to operate, good binding effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
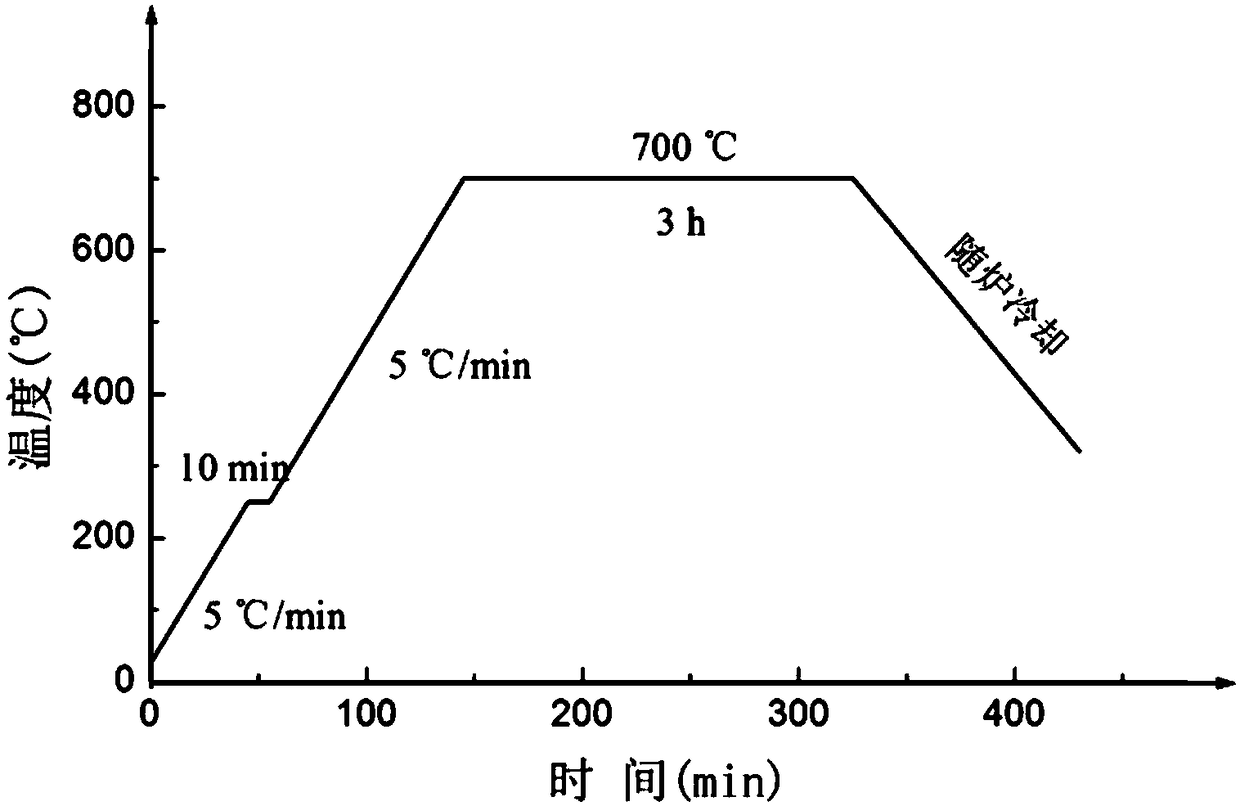
[0029] Embodiment 1: the preparation of the tungsten / copper layered composite material based on the surface nanometerization of the tungsten sheet, the steps are as follows:
[0030] Step 1. Pretreatment of tungsten sheet:
[0031] Take a tungsten sheet (purity ≥ 99.95wt%) with a size of 25×20×0.1mm as the sample, and use 800#, 1000# and 1500# metallographic sandpaper to polish the sample in turn, and each time the sandpaper is changed, the The direction is rotated 90° until the previous grinding traces completely disappear, and finally only the grinding traces of 1500# sandpaper are left on the surface of the tungsten sheet; after the grinding is completed, the polished tungsten sheet is polished with 0.5 μm diamond polishing agent, and then polished in acetone and isophthalic acid respectively. Ultrasonic cleaning in propanol and methanol for 10 minutes to achieve the purpose of oil removal, and finally ultrasonic cleaning in absolute ethanol and ultrapure water for 10 minut...
Embodiment 2
[0055] Embodiment 2, the preparation of the tungsten / copper layered composite material based on the nanometerization of the surface of the tungsten sheet. The surface of the composite material is smooth and bright, and the thickness of the copper metal layer is about 6.2 μm. After the scratch test and thermal shock test, the copper metal layer has no peeling and peeling phenomenon, and the bonding force with the tungsten substrate is good.
Embodiment 3
[0056] Example 3, the preparation of tungsten / copper layered composite material based on nano-sized tungsten sheet surface, the steps are basically the same as in Example 1, the only difference is: in step 4, the electroplating temperature is controlled at 60°C, and the final tungsten / copper The surface of the layered composite material is flat and bright, and the thickness of the copper metal layer is about 5.2 μm. After the scratch test and thermal shock test, the copper metal layer has no peeling and peeling phenomenon, and the bonding force with the tungsten substrate is good.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Average pore size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com