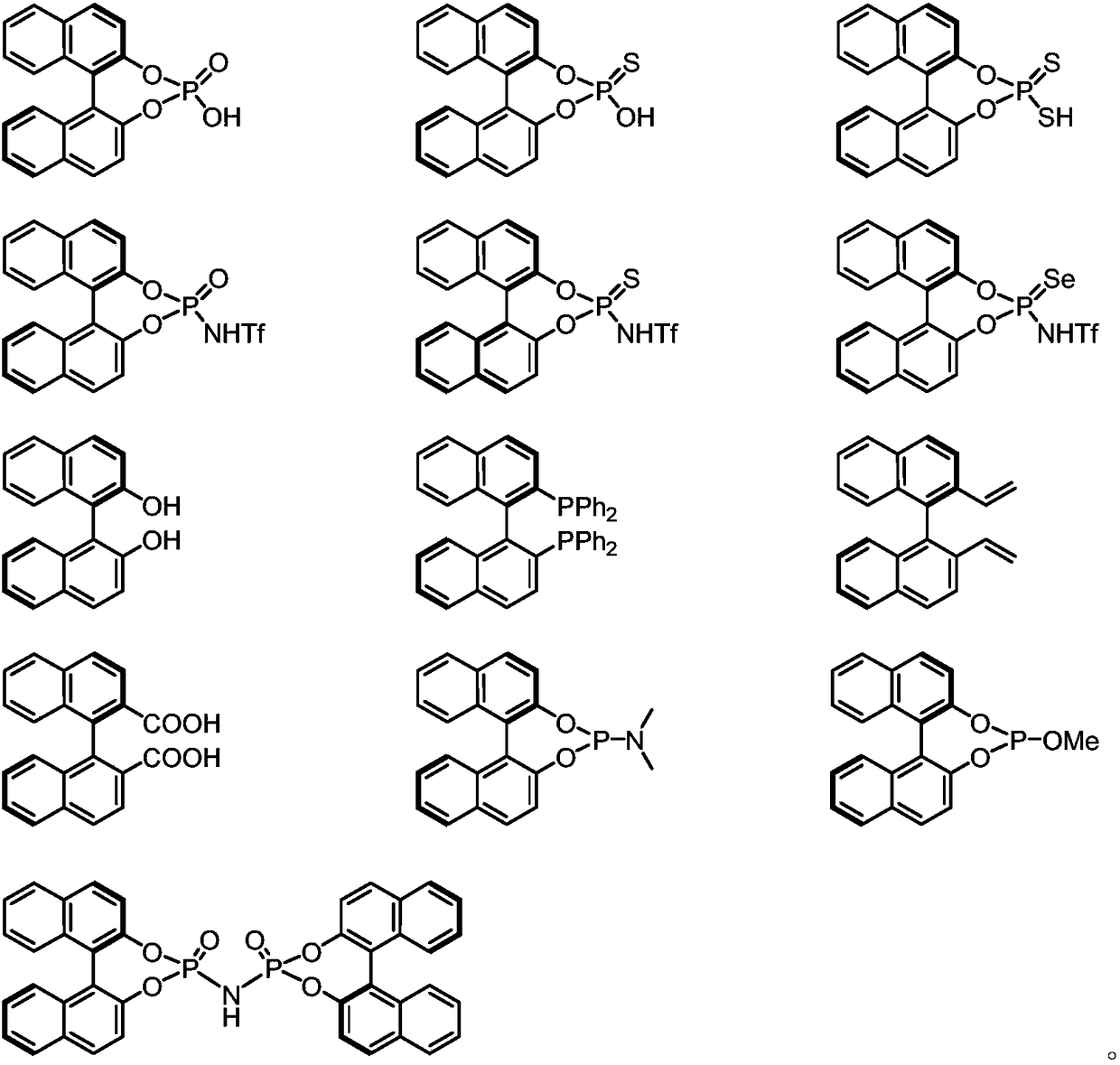
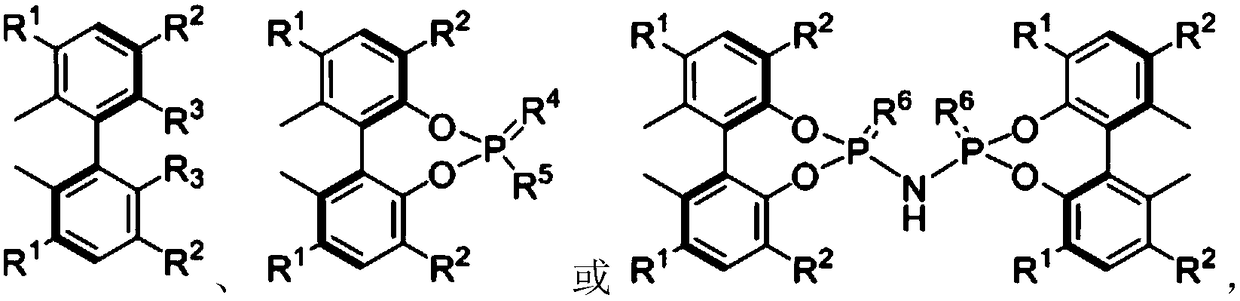
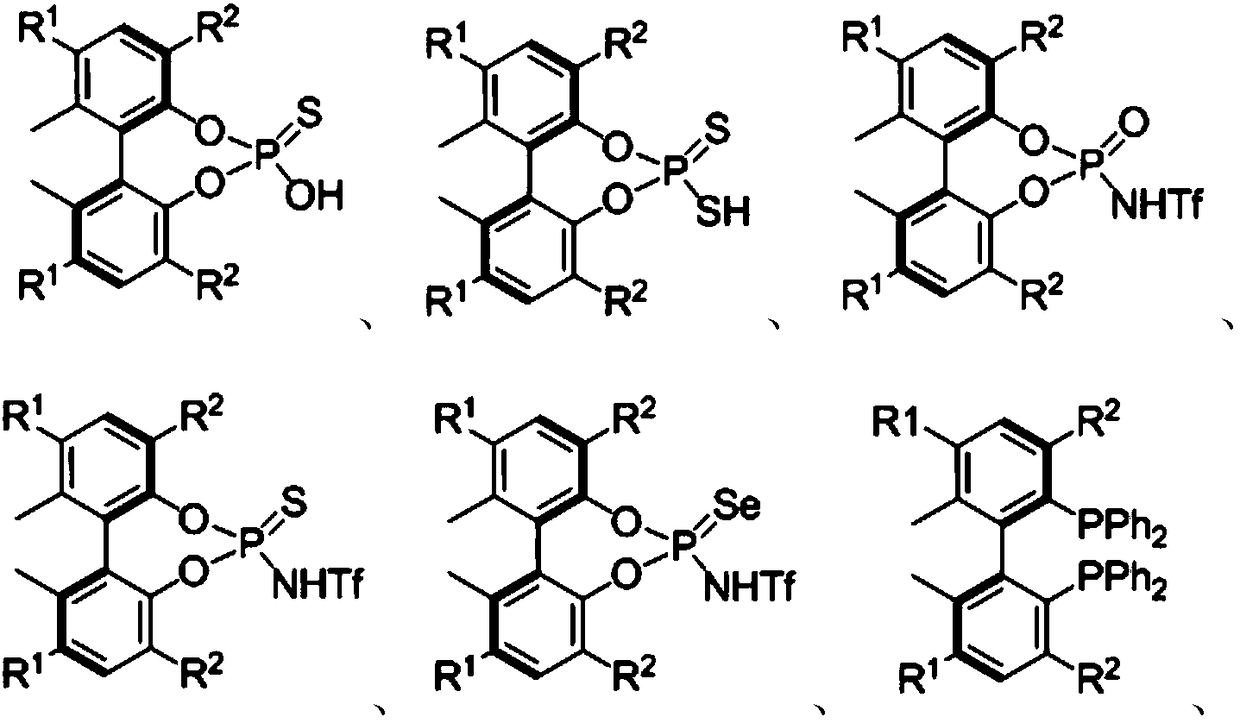
Chiral compounds containing biphenyl skeleton, and preparation method and applications thereof
A chiral compound and biphenyl technology are applied in the field of chiral compounds containing biphenyl skeleton and their preparation, and can solve the problems of complex structure, many synthesis steps, and complicated preparation process of chiral binaphthalene compounds.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] The preparation method of class A compound is as follows:
[0064]
[0065] (1) Under nitrogen protection conditions, weigh 12g of compound 1, 12.50g of phenylboronic acid, 25g of potassium carbonate, 600mg of [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride (100mg, 0.12mmol) in a 500mL two-neck reaction flask, after three times of evacuation and nitrogen supplementation, add 300mL of a mixed solvent of ethylene glycol dimethyl ether and water (V / V, 2:1), heat to 95°C and react under nitrogen protection After 10 hours, cool to room temperature, add dichloromethane to dissolve after removing the solvent, remove the insoluble matter by suction filtration, wash the organic phase with saturated sodium chloride, spin dry, and separate the crude product through a column (petroleum ether: dichloromethane=5 : 1), to obtain 9.88g compound 2, productive rate 83%.
[0066] (2) Add 4.78g of compound 2 into a 250mL two-neck round bottom flask, and then inject 70mL of d...
Embodiment 2
[0073] The preparation method of class B compound is as follows:
[0074]
[0075] Weigh 1.5g of compound 5 and 643mg of phosphorus pentasulfide in 20mL of dry dichloromethane, reflux at 150°C for 5h, remove the solvent under reduced pressure after the reaction, extract the reaction solution with dichloromethane and water, combine the organic phases, spin dry, The crude product was separated by column to obtain 1.38 g of compound B with a yield of 78%.
[0076] The analysis result of compound B is: 1 H NMR (CDCl 3 , 400MHz): δ8.14-8.09 (m, 12H), 7.84-7.55 (d, 4H), 7.47-7.40 (d, 4H), 7.38 (s, 2H), 1.62 (s, 6H).
Embodiment 3
[0078] The preparation method of C compound is as follows:
[0079]
[0080] (1) Weigh 2 g of compound 5 and dissolve in 30 mL of dry dichloromethane, add 1 mL of freshly distilled thionium trichloride at one time, and then slowly add 2 mL of anhydrous triethylamine. After stirring at room temperature for 20 h, the solvent was removed under reduced pressure, and the crude product was separated by column to obtain 1.89 g of compound 7 with a yield of 82%.
[0081] (2) Weigh 1.89g of compound 7 and dissolve it in 30mL of dry mixed solution of dichloromethane and acetonitrile (V / V, 1:2), add 771mg of 4-dimethylaminopyridine, 2mL of triethylamine and 1.18g of Trifluoromethanesulfonamide was refluxed for 10h. After the reaction was completed, water was added to quench, and the reaction liquid was extracted with dichloromethane and water. The organic phases were combined, dried, and the solvent was removed under reduced pressure. The crude product was separated by column to obta...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com