Carbon-based supramolecular recognition material and preparation method thereof and application of carbon-based supramolecular recognition material in adsorption and separation of Cs
A supramolecular and carbon-based technology, applied in chemical instruments and methods, other chemical processes, alkali metal oxides/hydroxides, etc., can solve the problem of low selectivity and adsorption capacity, small particle size of supramolecular materials, organic reagents Problems such as large amount of use, to achieve the effect of high partition coefficient and selectivity, simple synthesis method, and high practical value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] Such as figure 1 As shown, the preparation method of the carbon-based supramolecular recognition material provided by the embodiment of the present invention includes the following steps:
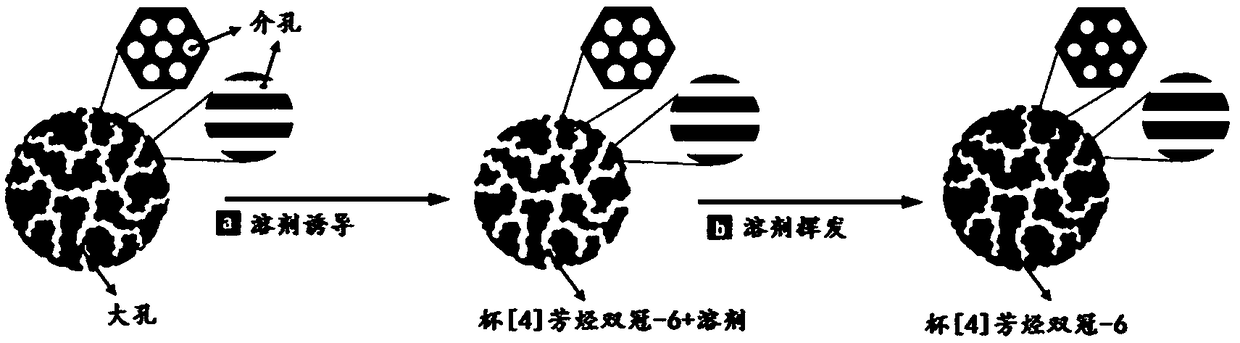
[0036] S101: Polymerize the polymer in millimeter-scale macroporous-mesoporous carbon spheres to form a carbon-based carrier, and the mass percentage of the polymer in the carbon-based carrier is 0-10%;
[0037] S102: Using carbon-based carrier and calix[4]arene double crown-6 as raw materials, a carbon-based supramolecular recognition material was prepared by vacuum solvent evaporation-induced self-assembly method.
[0038] The preparation method of the carbon-based carrier of the present invention is:
[0039] (1) Concentrated nitric acid was added to millimeter-scale macroporous-mesoporous carbon spheres, reacted at 120°C for 4h, cooled to room temperature, washed until neutral, and dried at 80°C for 4h.
[0040] (2) Under vacuum conditions, add polymer monomers and correspondi...
Embodiment 1
[0053] Embodiment 1 carbon-based supramolecular recognition material
[0054] Add 5g of millimeter-sized macroporous-mesoporous carbon spheres to 4mL of concentrated nitric acid, react at 120°C for 4h, cool to room temperature, wash until neutral, and dry at 80°C for 4h;
[0055] 0.212 g m / p-formylstyrene, 0.0376 g m / p-divinylbenzene, 0.675 g methyl Sodium benzoate, 0.426g dioctyl phthalate, 0.035g 1,1-dicyclohexylamine-1-carbonitrile mixture and 0.035g a,a-azobisisobutyronitrile, and spin at room temperature Steam for 90 minutes, then raise the temperature to 50°C for 120 minutes. and in N 2 Under protective conditions, move to a three-necked flask, continue to react at 90°C for 24h, and then cool to room temperature.
[0056] Wash and filter the above product with acetone and methanol, repeat three times, put it into a vacuum oven and dry at 50°C for 24 hours to obtain the MMCs-P carbon-based material, weigh 2.0g of MMCs-P and add 80mL of methanol, shake at constant tempe...
Embodiment 2
[0057] The preparation of embodiment 2 carbon-based carrier
[0058] Add 5g of millimeter-sized macroporous-mesoporous carbon spheres to 4mL of concentrated nitric acid, react at 120°C for 4h, cool to room temperature, wash until neutral, and dry at 80°C for 4h;
[0059] Under vacuum conditions, with 1,2,3-trichloropropane and m-xylene as solvents, m / p-formylstyrene, m / p-divinylbenzene, sodium methylbenzoate, Dioctyl phthalate, 1,1-dicyclohexylamine-1-carbonitrile mixture and a,a-azobisisobutyronitrile, and rotate at room temperature for 90min, then raise the temperature to 50°C to continue 120min. and in N 2 Under protective conditions, move to a three-necked flask, continue to react at 90°C for 24h, and then cool to room temperature.
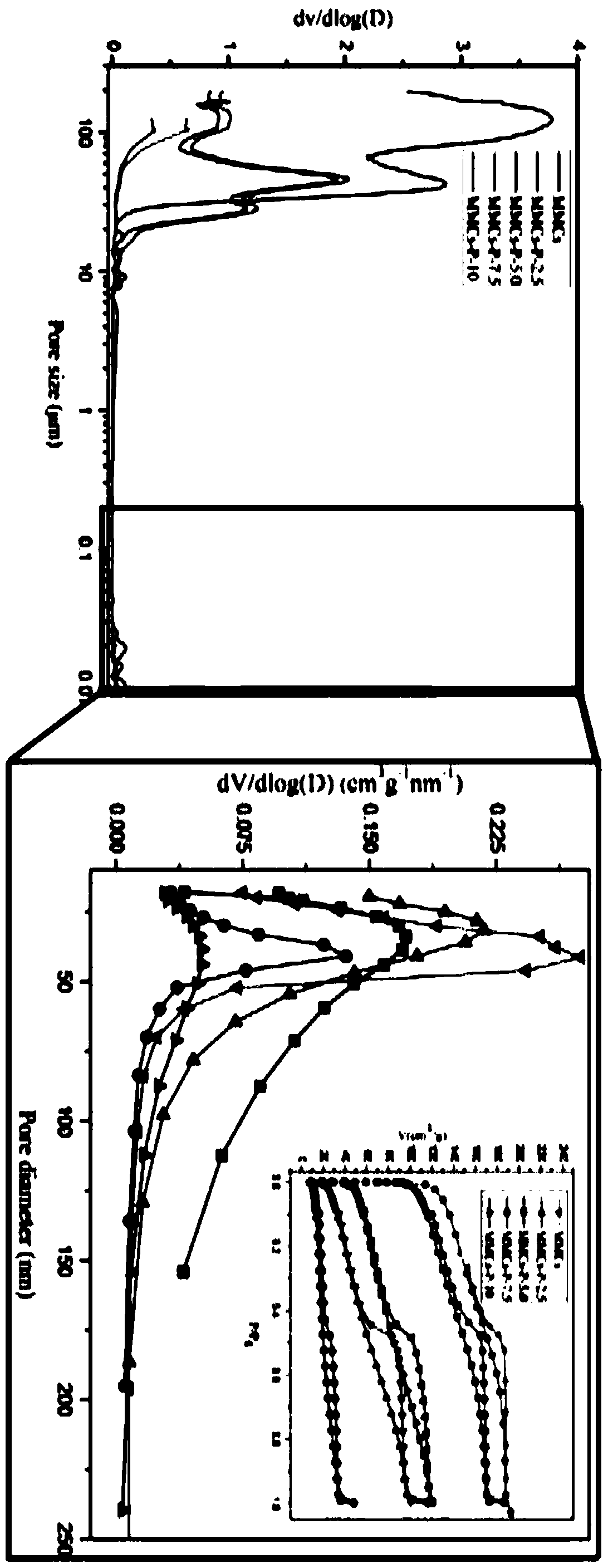
[0060] Wash and filter the above product with acetone and methanol, repeat three times, put it in a vacuum oven and dry it at 50°C for 24 hours to obtain MMCs-P-x carbon-based material, where x represents the mass of styrene-divinylbenzene ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com