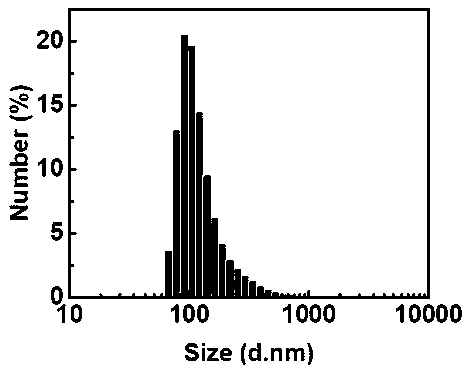
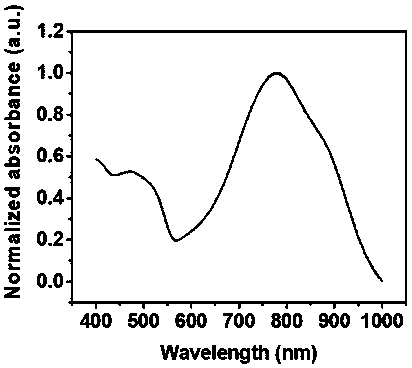
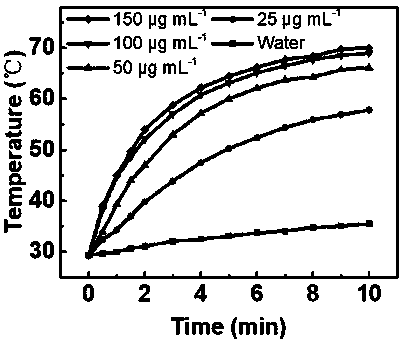
Tellurene-based conjugated polymer and its synthesis method and application
A conjugated polymer, telluride-based technology, applied in the field of telluride-based conjugated polymers and their synthesis, can solve problems such as expensive environment, patient danger, and harm, and achieve remarkable effects, strong absorption, and excellent biocompatibility. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0069] The synthetic method of tellurium phenyl conjugated polymer, the step comprises:
[0070] (1) under the protection of an inert gas, under the catalysis of a palladium catalyst, the organic acid, inorganic base, ligand, intermediate 1 and intermediate 2 are all placed in an organic solvent for reaction, and the reaction temperature is 100 to 120 ° C, The reaction time is 14-72 hours to obtain a mixture; wherein, the molar ratio of palladium catalyst, organic acid, inorganic base, ligand, intermediate 1, and intermediate 2 is 0.1-0.3:0.3:2.5:0.1:1:1, The ratio of intermediate 1 (amount of substance, unit: mol) to organic solvent (volume, unit: liter) is 0.01-0.5:1; the palladium catalyst is selected from palladium acetate or tris(dibenzylideneacetone) dipalladium; The organic acid is pivalic acid or 1-adamantanecarboxylic acid; the inorganic base is selected from sodium carbonate, potassium carbonate and cesium carbonate; One of tert-butylphosphine and tricyclohexylphosp...
Embodiment 1
[0083] Synthesis of embodiment 1 tellurene-based conjugated polymer
[0084] With 0.056 mmoles of intermediate 2 (connecting tellurophene), 0.056 mmoles of R is the intermediate 1 (2-octyl dodecyl dibromonaphthalimide) of 2-octyl dodecyl, 0.14 mmol of cesium carbonate (2.5 equivalent), 0.0168 mmol of pivalic acid (0.3 equivalent), 0.0056 mmol of tricyclohexylphosphine (0.1 equivalent), were added to 0.1 ml of toluene solution. After nitrogen sparging for 20 minutes, 0.0056 mmol of tris(dibenzylideneacetone)dipalladium (0.1 equiv) was added and bubbled for another 10 minutes. After sealing, react at 120 degrees Celsius for 60 hours. After cooling, 0.5 milliliters of the mixture was added dropwise to 200 milliliters of methanol, a solid was precipitated, and Soxhlet extraction was performed in the order of acetone, n-hexane and chloroform, and the heating temperature was 100 degrees Celsius. The volumes of acetone, n-hexane and chloroform are all 200 ml. The obtained polymer ...
Embodiment 2
[0085] Synthesis of embodiment 2 tellurene-based conjugated polymers
[0086] With 0.056 millimoles of intermediate 2 (even tellurophene), 0.056 millimoles of R is 2-ethyloctyl intermediate 1 (2-ethyloctyl dibromonaphthalimide), 0.14 millimoles of Potassium carbonate (2.5 equivalent), 0.0168 mmol of 1-adamantanecarboxylic acid (0.3 equivalent), 0.0056 mmol of tris(3-methoxyphenyl)phosphine (0.1 equivalent), were added to 5 ml of toluene solution. After nitrogen sparging for 20 min, 0.0112 mmol of palladium acetate (0.2 equiv) was added and sparged for an additional 10 min. After sealing, react at 100 degrees Celsius for 14 hours. After cooling, 0.5 ml of the mixture was added dropwise into 400 ml of methanol, and a solid was precipitated, which was subjected to Soxhlet extraction in the order of acetone, n-hexane and chloroform. The temperature of soot is 100 degrees centigrade, and the volume of acetone, normal hexane and chloroform added in the bottle mentioned above is 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| photothermal conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com