Facultative carbon nitride hydrophilic enrichment material as well as preparation method and application thereof
A kind of carbon nitride, hydrophilic technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problem that non-glycopeptides cannot be effectively removed, affect glycopeptide mass spectrometry response, glycopeptide interference, etc. problem, to achieve the effect of low production cost, large enrichment, easy and controllable operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Embodiment 1, preparation of glycopeptide enrichment material
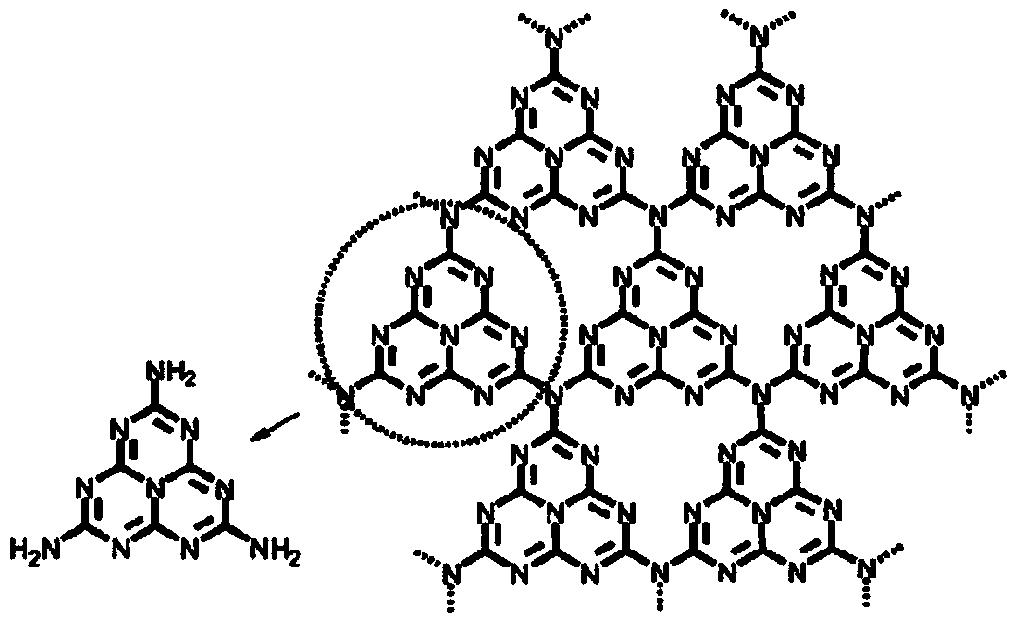
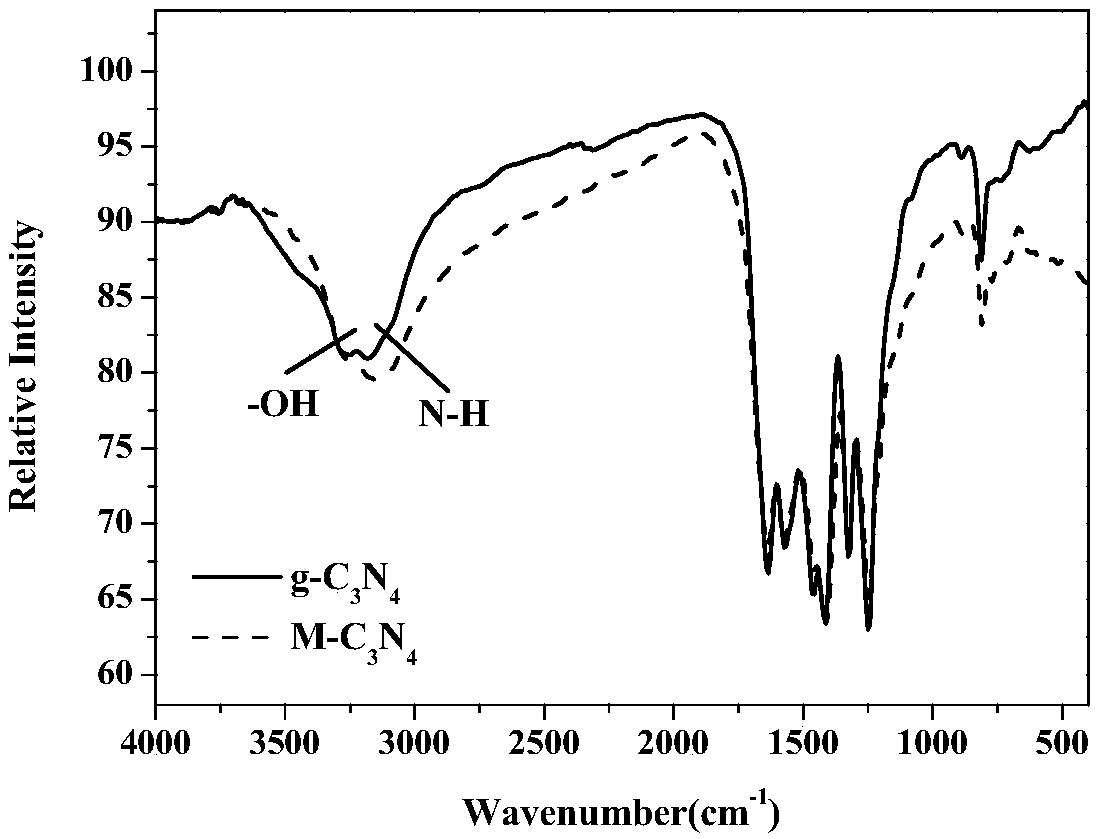
[0060] (1) Melamine C 3 N 3 (NH 2 ) 3 The solid is calcined in a tube furnace at 600°C under air conditions, the heating rate is 3°C / min, and the temperature is kept for 2 hours. After natural cooling, it is homogenized to obtain a light yellow powder;
[0061] (2) 200 mg of the above powder is dispersed in 300 mL of deionized water to form a dispersion liquid. In a temperature-controlled microwave reactor at 95 ° C, 200 W magnetic stirring at 300 rpm, add condensed water to reflux, microwave reaction for 12 hours, and cool to room temperature after the reaction, the obtained The dispersion was centrifuged at 1000 rpm for 2 minutes, the precipitate was discarded, and the upper suspension was retained to obtain a milky white suspension dispersion;
[0062] (3) the above-mentioned thin layer graphite phase carbon nitride (M-C 3 N 4 ) dispersion, add 100μL 20mMHAuCl under magnetic stirring 4 Solution, u...
Embodiment 2
[0078] Embodiment 2, human immunoglobulin (IgG) glycopeptide enrichment experiment
[0079] (1) Human IgG was dissolved in 50mM ammonium bicarbonate solution to make a concentration of 1mg·mL -1 protein solution. Direct heat denaturation at 95°C for 10 min. After reduction with 10mM dithiothreitol solution at 56°C for 1h, it was alkylated with 50mM iodoacetamide solution for 1h in the dark. Add trypsin to the solution at an enzyme / protein ratio of 1:50 (w / w), and incubate at 37°C for 16 hours to enzymatically digest the standard protein sample. All protein hydrolyzate was desalted by Sep-Pak desalting column, freeze-dried in a vacuum refrigerated centrifuge, and stored at -20°C for future use.
[0080] (2) Dissolve the IgG enzyme-cleaved peptide in 50-500 μL of a solution containing 80%-90% acetonitrile and 0.2% trifluoroacetic acid, and add it at a peptide / enrichment material ratio of 1:50 (w / w) for implementation The facultative carbon nitride hydrophilic material (L-Cys...
Embodiment 3
[0087] Embodiment 3, human urine glycopeptide enrichment experiment
[0088] (1) Use a 50mL centrifuge tube to collect human morning urine (midstream urine) and mark it. First centrifuge at 3000g for 15min, then centrifuge at 12000g for 15min. Keep the supernatant and discard the pellet. 10mL of urine was added to 40mL of pre-cooled acetone, mixed upside down and placed in the refrigerator (-20 degrees) overnight. Centrifuge at 5000g for 5min, discard the supernatant and keep the precipitate. Blow off excess acetone using nitrogen gas. After redissolving with 8M UA, it was then transferred to a 30KD ultrafiltration tube.
[0089] (2) After reducing with 20mM dithiothreitol solution at 37°C for 4h, centrifuge at 13000 rpm for 15min, then use 50mM iodoacetamide solution for alkylation treatment in the dark for 30min, centrifuge at 3000rpm for 15min, and again React with 20mM dithiothreitol solution for 15min, and centrifuge at 13000 rpm for 15min. Add 200 μL of 50 mM ammon...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com