Recombinant virus and application thereof in preparation of medicine for preventing or treating renal fibrosis
A kidney fibrosis and recombinant virus technology, applied in the field of genetic engineering, can solve the problems of not showing additive effect, unable to completely block the overexpression of TGFβ1, and unable to completely block the progress of renal failure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
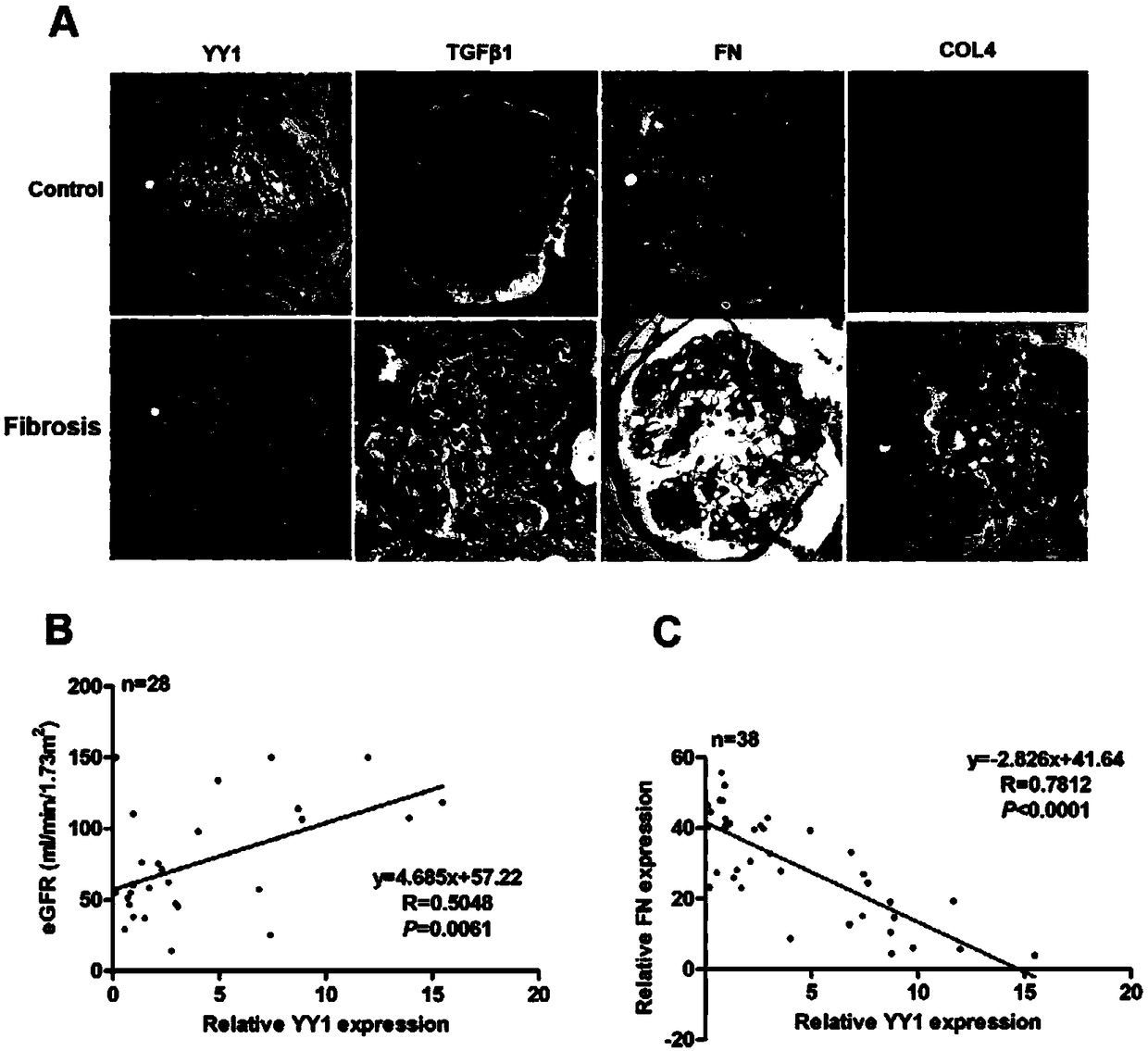
[0036] The Specimen Bank of Shanghai Sixth People's Hospital collected renal specimens from patients who signed the informed consent form and were pathologically diagnosed as renal fibrosis between 2015 and 2017. Immunohistochemical staining was performed on paraffin sections of renal biopsies , the specific steps are as follows: perform paraffin section, dewax to water with xylene and 95% alcohol; then, rinse with distilled water, and then remove endogenous peroxidase with 3% hydrogen peroxide aqueous solution (37°C for 30min); Then, wash with double distilled water and TBST, and then use sodium citrate solution for microwave repair; after the solution is cooled, normal sheep serum is blocked for 20 minutes, and primary antibody is added, incubated at 37°C for one hour, and overnight at 4°C; the next day , After the slices were equilibrated at room temperature, washed three times with TBST, added secondary antibody and incubated at 37°C for one hour, after color development by...
Embodiment 2
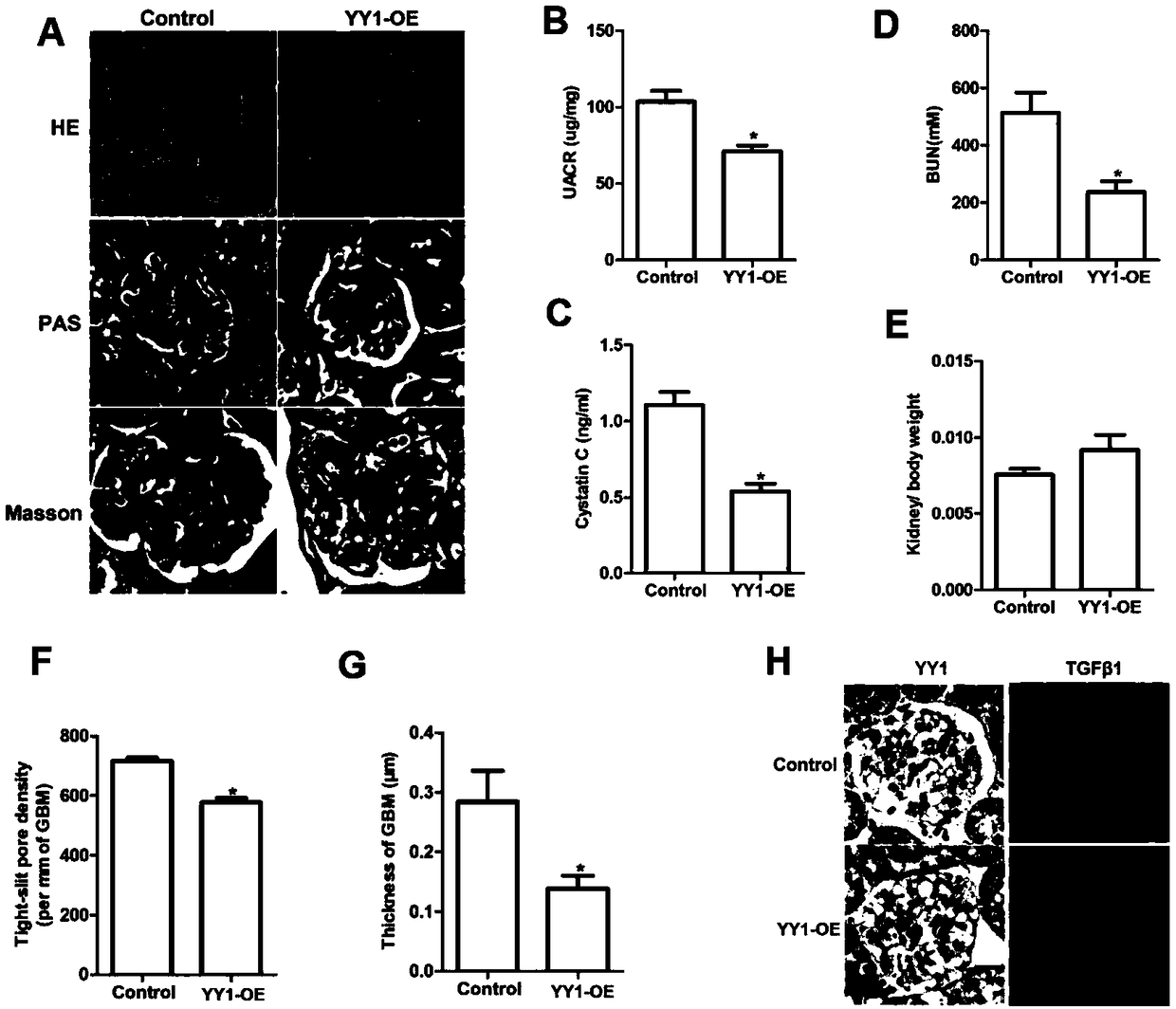
[0039] Construction of YY1 Overexpression Recombinant Virus
[0040] see Figure 4 , the inventors used pHBAAV-CMV-MCS-3flag-T2A-ZsGreen, an adeno-associated virus vector, and used two restriction enzymes, BamH I and Kpn I, to digest with CutSmart buffer at 37°C for 1 hour. The gel was cut and recovered, and the purified linear vector was further obtained. Meanwhile, YY1 (SEQ ID NO.1) was amplified by PCR, the forward primer used was 5'-CCATAGAAGACACCGGGATCCGCCACCATGGCCTCGGGCGACACC-3'(SEQ ID NO.4), and the reverse primer used was 5'-GTAGTCGTTAATTAAGGTACCCTGGTTGTTTTTGGCCTTAGCATGT- 3' (SEQ ID NO.5); after PCR, the DNA was recovered to obtain a purified CDS fragment of linear YY1. Next, ligate the prepared linear vector with the CDS fragment of the linear YY1 (LigationHigh enzyme, 16°C for 1h), and then perform transformation, which specifically includes the following steps: add 10ul of the ligation product to Beijing Quanshijin Biotechnology Co., Ltd. Stable3 competent cells ...
Embodiment 3
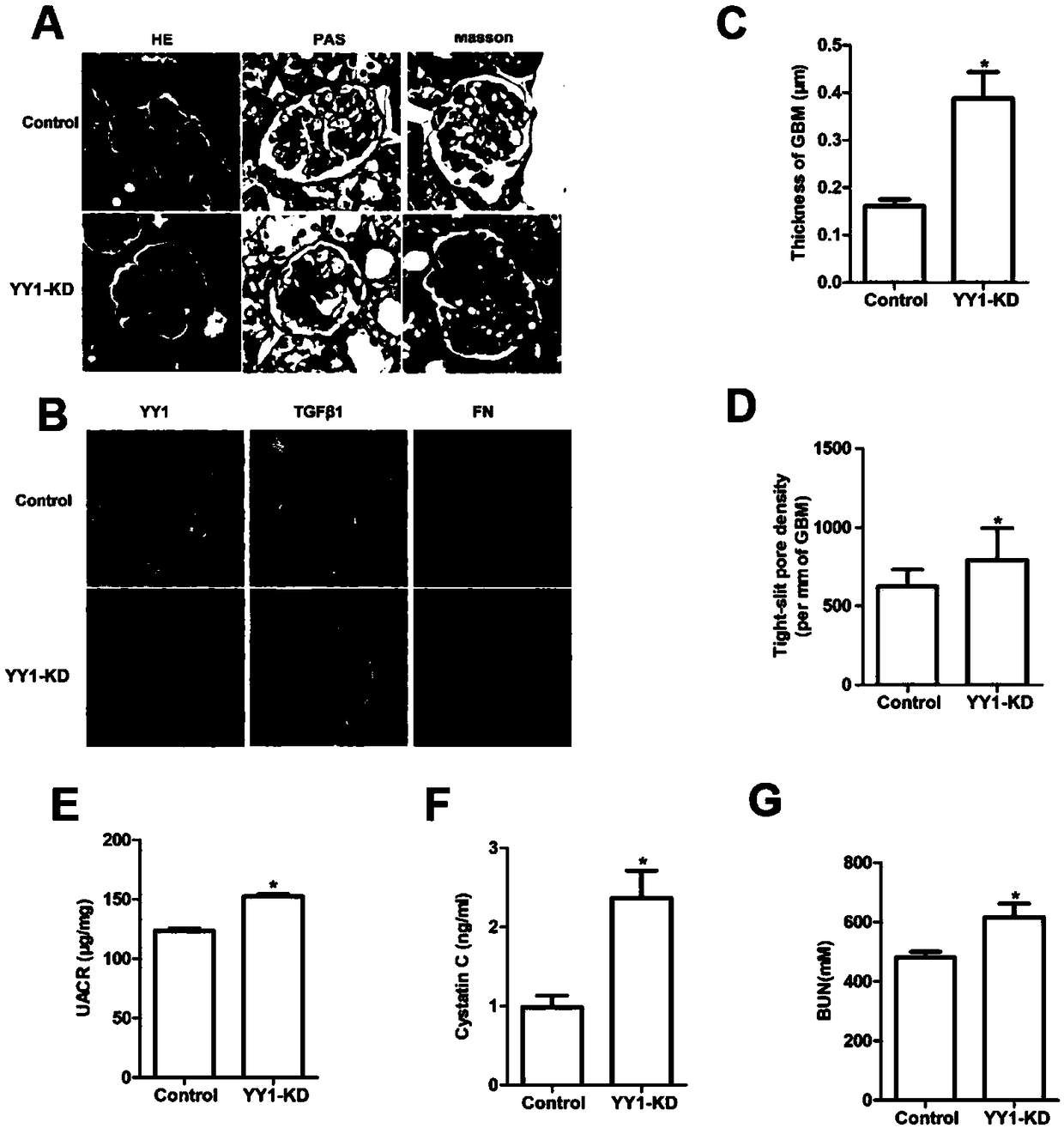
[0043] Kidney-specific knockout of YY1 experiment
[0044] 4-week-old C57BL6 / J YY1 flox / flox Male rats were fed with a high-fat diet for 6 weeks and injected intraperitoneally with streptozotocin (STZ, 40 mg / kg), and injected every other day. Fasting blood glucose was measured after a total of three times. Male rats greater than 16.7 mmol / L were randomly divided into two groups. group, 12 rats in each group; one week later, the kidneys were injected with AAV-cre virus in situ: after the mice were anesthetized with pentobarbital sodium, the abdomen was opened, and the left kidney was carefully separated, and three points were taken from the lower border of the left renal cortex. Injection (10ul per point 1*10 12 AAV-cre virus); the control group was treated in the same way, the wound was sutured after injection of the same dose of AAV-GFP control virus, and the mice were sacrificed after continuing to feed for 12 weeks. During the feeding period of the mice, the body weight a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com