Preparation method and application of recombinant allosteric collagenase
A technology of collagenase and collagenase, which is applied in the field of purification of recombinant allosteric collagenase, can solve the problems of affecting the expression of protein solubility, inability to enter the market, and low protein purity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Example 1 Construction of Recombinant Allosteric Collagenase Strains
[0053] Instruments and materials
[0054] The ColH allosteric collagenase gene sequence was artificially synthesized. pET-30a(+), host bacteria BL21(DE3), BL21(DE3)playS, and Transetta were purchased from Merck, and endonuclease was purchased from Thermo.
[0055] experimental method
[0056] figure 1 The E451D single point mutation ColH allosteric collagenase sequence is described. figure 2 The E451D single point mutation ColH allosteric collagenase protein sequence is described. The synthesized plasmid and pET-30a(+) empty vector were digested with NdeI / XhoI, detected by electrophoresis, and the target fragment and vector fragment were recovered by slicing gel. Ligate the recovered two fragments with T4 DNA ligase, transform 10ul of the ligation product into 100ul of competent cells, plate and pick a single clone, and the clone with the correct sequencing result is the target strain.
[0057]...
Embodiment 2
[0058] Embodiment 2 Recombinant Allosteric Collagenase Strain Fermentation
[0059] Instruments and materials
[0060] BIOFLO 610 65.0L fermentation tank was purchased from Eppendorf Company, high-speed refrigerated centrifuge was purchased from Thermo Company, working seed lot, peptone and yeast extract were purchased from OXID Company, and various reagents were purchased from Sinopharm Chemical Reagent Company.
[0061] experimental method
[0062] After the shake flask seeds are cultivated overnight, they are inoculated into the seed tank in a suitable state, and after a certain period of cultivation, the seed liquid is injected into the production tank connected to it.
[0063] Cultivate at 37°C for a certain period of time, the production medium formula is peptone 13.5051g / L, yeast powder 7g / L, magnesium sulfate 0.4g / L; 4 hours after inoculation in the production tank, add IPTG with a final concentration of 0.5mM for induction, and the induction time is 7-8 Hours, feedi...
Embodiment 3
[0065] Embodiment 3 Purification method of recombinant allosteric collagenase
[0066] Instruments and materials
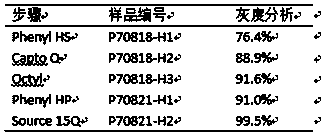
[0067] Fillers such as Capto Phenyl HS, Capto Q, CaptoOctyl, and Phenyl HP were purchased from GE Company, AktaPurifier chromatography system (GE Company), and hollow fiber column ultrafiltration system (Pall).
[0068] experimental method
[0069] 1) Bacterial harvest and clarification
[0070] After the fermentation, the bacterial cells are collected by centrifugation; further, the bacterial cells can be collected by membrane treatment and other methods after amplification. The fresh bacteria can be preserved by freezing, or directly crushed to enter the next step. The bacteria to be crushed are resuspended uniformly in Tris buffer at a resuspension concentration of 10-20%, crushed by a high-pressure homogenizer at 600-700 bar pressure, passed through a high-pressure valve for 3 times, and the temperature is controlled at 2-8°C during the crushing process.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com