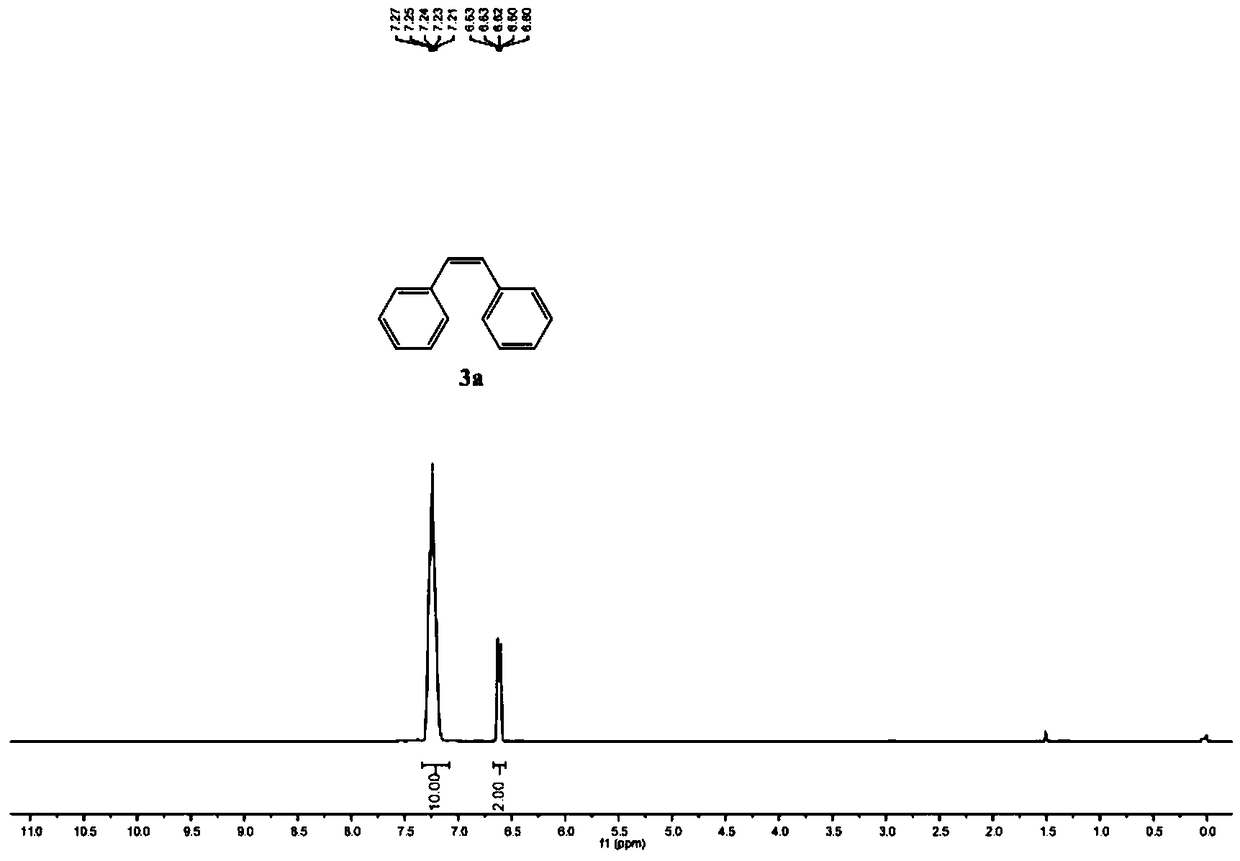
Method for catalytic-selectively synthesizing Z- and E-alkene through alcohol supply hydrogen iridium controlled by ligand
A selective and ligand-based technology, applied in chemical instruments and methods, hydrogenation to hydrocarbons, organic chemistry, etc., can solve the problem that it is difficult to realize the selective synthesis of Z- and E-olefins at the same time, which is unfavorable for large-scale industrial production and difficult to achieve. The problem of obtaining trans olefins, etc., achieves the effects of good catalytic effect, convenient post-processing procedures, and wide source of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Synthesis of (E)-1,2-diphenylethylene:
[0043]
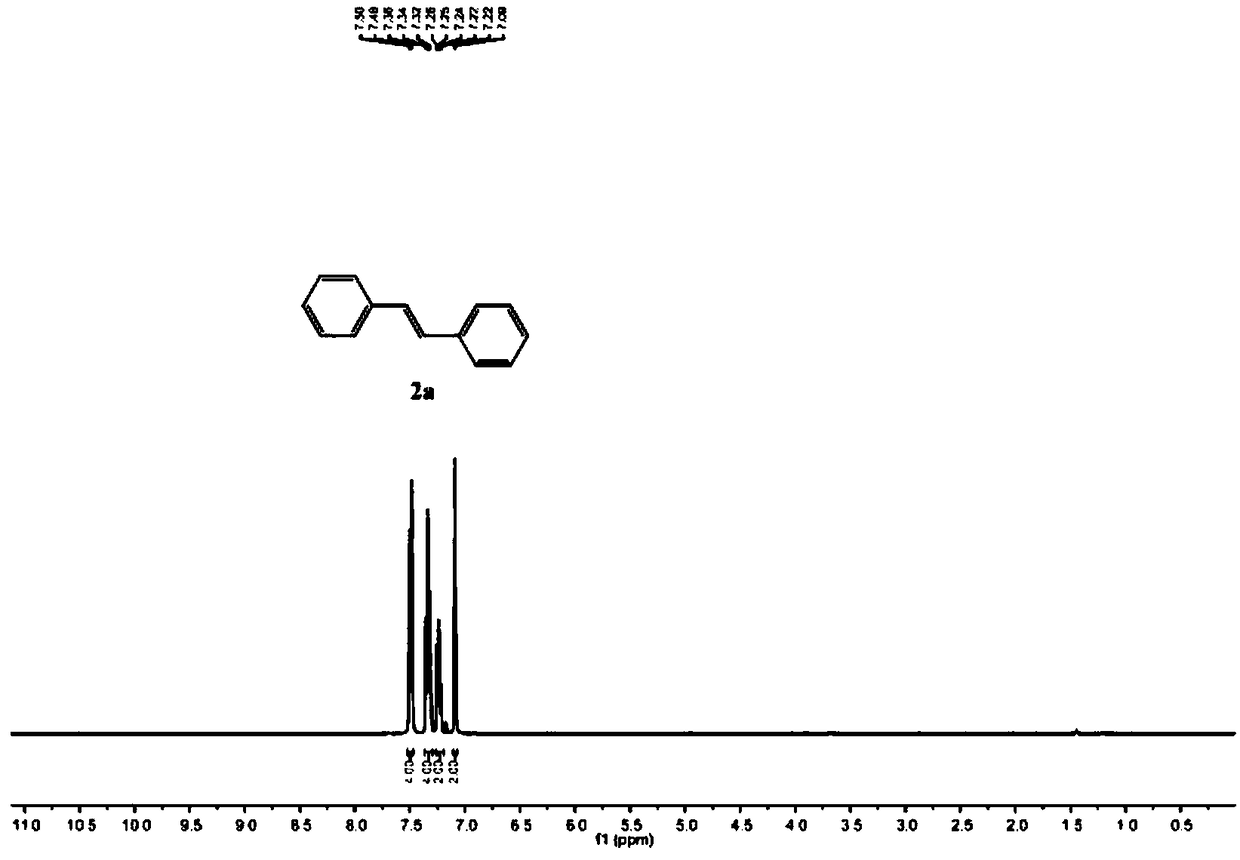
[0044] Add raw material 1a (0.20mmol) and EtOH (4mmol, 232L) successively in reaction flask, [Ir(cod)Cl] 2 (10μmol, 8.4mg), DPPE (0.04mmol, 15.9mg) and tetrahydrofuran (1.5mL), in a nitrogen atmosphere, stirred at 120°C for 22 hours, cooled to room temperature, diluted with ethyl acetate (5mL), saturated saline (5 mL), the organic phase was dried with anhydrous magnesium sulfate, spin-dried and then column chromatographed with (n-hexane) as the eluent to obtain 33 mg of product 2a as a white solid, with a yield of 92%. 1 H NMR (400MHz, CDCl 3 ):7.49(d, J=8.0Hz, 4H), 7.34(t, J=8.0Hz, 4H), 7.26–7.22(m, 2H), 7.09(s, 2H); 13 C NMR (100MHz, CDCl 3 ) 137.4, 128.8, 127.7, 126.6.
Embodiment 2
[0046] Synthesis of (E)-1-bromo-4-styrylbenzene:
[0047]
[0048] Add raw material 1b (0.20mmol) and EtOH (4mmol, 232L) successively in reaction flask, [Ir(cod)Cl] 2 (10μmol, 8.4mg), DPPE (0.04mmol, 15.9mg) and tetrahydrofuran (1.5mL), in a nitrogen atmosphere, stirred at 120°C for 22 hours, cooled to room temperature, diluted with ethyl acetate (5mL), saturated saline (5 mL), the organic phase was dried with anhydrous magnesium sulfate, spin-dried and then column chromatographed, the eluent was (n-hexane), and the product 2b was obtained as 44 mg of a white solid, with a yield of 85%. 1 H NMR (400MHz, CDCl 3 ):7.48–7.43(m,4H),7.35–7.31(m,4H),7.23(d,J=8.0Hz,1H),7.07(dd,J=24.0,12.0Hz,2H); 13 C NMR (100MHz, CDCl 3 ) 137.1, 136.4, 131.9, 129.6, 128.9, 128.1, 128.1, 127.6, 126.7, 121.5.
Embodiment 3
[0050] Synthesis of (E)-1-chloro-4-styrylbenzene:
[0051]
[0052] Add raw material 1c (0.20mmol) and EtOH (4mmol, 232L) successively in reaction flask, [Ir(cod)Cl] 2 (10μmol, 8.4mg), DPPE (0.04mmol, 15.9mg) and tetrahydrofuran (1.5mL), in a nitrogen atmosphere, stirred at 120°C for 22 hours, cooled to room temperature, diluted with ethyl acetate (5mL), saturated saline (5 mL), the organic phase was dried with anhydrous magnesium sulfate, spin-dried and then column chromatographed, the eluent was (n-hexane), and the product 2c was obtained as 36 mg of a white solid, with a yield of 84%. 1 H NMR (400MHz, CDCl 3 ):7.50(d, J=8.0Hz, 2H), 7.44(d, J=8.0Hz, 2H), 7.38-7.25(m, 5H), 7.07(dd, J=20.0, 16.0Hz, 2H); 13 C NMR (100MHz, CDCl 3 ) 137.1, 136.0, 133.3, 129.5, 129.0, 128.9, 128.0, 127.8, 127.5, 126.7.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com