Secretory expression method of carboxypeptidase B
A technology of secreted expression and carboxypeptidase, applied in peptidases, biochemical equipment and methods, enzymes, etc., can solve the problems of low CPB yield, low renaturation efficiency, limited application, etc., achieve easy recovery and purification, and promote solubility. expressive effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: Construction of recombinant expression vector
[0043] (1) Signal peptide site-directed mutation
[0044] Plasmid pET20b (with signal peptide PelB) and synthetic signal peptides OmpA, Endoxylanase, OmpT were used as templates, and primers OA-DB-F and OA-DB-R, PB-DB-F and PB-DB- R, End-DB-F and End-DB-R, OT-DB-F and OT-DB-R for PCR;
[0045]PCR reaction system (200 μL): 100 μL of 2×super Pfu PCR Master Mix, 2 μL of each template, 1 μL of upstream and downstream primers, sterilized ddH 2 O 94 μL;
[0046] PCR amplification conditions: pre-denaturation at 95°C for 5min; denaturation at 94°C for 30s, annealing at 59°C for 30s, extension at 72°C for 2min, 30 cycles; extension at 72°C for 10min.
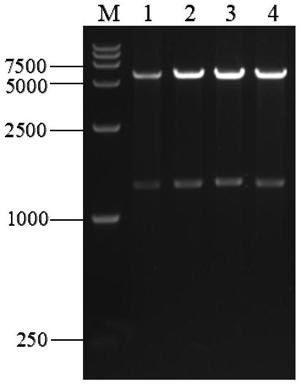
[0047] After the PCR product was recovered with a DNA purification kit, DpnI was added to digest the template, after purification, it was ligated with T4 DNA ligase overnight at 16°C, and 10 μL of the ligated product was transformed into the cloning host JM109 for c...
Embodiment 2
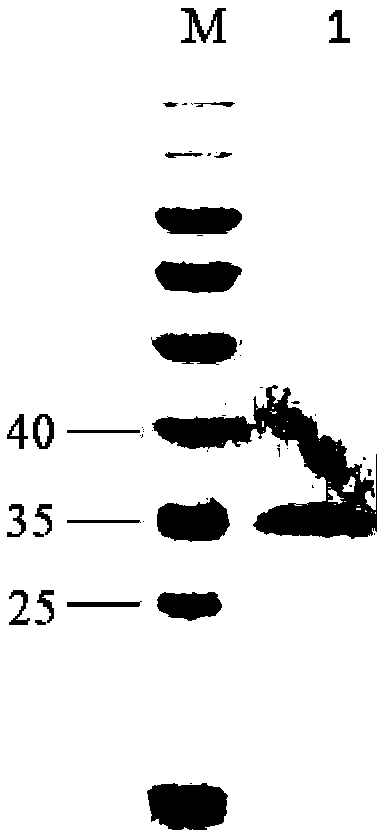
[0100]Example 2: Expression and purification of carboxypeptidase B in Escherichia coli
[0101] 1. Seed culture: Pick a small amount of strains preserved in glycerol tubes, streak them on LB plates (containing 100 μg / mL ampicillin Amp), and culture them overnight in a 37°C incubator. On the next day, single clones were picked, transferred to 15 mL of LB culture medium (containing 100 μg / mL ampicillin Amp), and cultured overnight at 37° C. on a shaker at 200 rpm.
[0102] 2. Shake flask culture: transfer to the secondary bottle with 2% inoculum, and continue to culture at 37°C until OD 600 When the concentration is 0.5-0.6, add IPTG with a final concentration of 0.1mM, and culture at 25°C for 30h.
[0103] 3. Enzyme hydrolysis of procarboxypeptidase B: centrifuge the induced expression medium at 12,000 rpm for 10 min, and the supernatant is the crude enzyme solution of procarboxypeptidase B, and measure the protein concentration by Coomassie brilliant blue method. Filter the ...
Embodiment 3
[0108] Embodiment 3: the enzyme activity assay of recombinant CPB
[0109] Assay buffer: 25mM Tris-HCl, containing 0.1mol / L NaCl (pH7.65)
[0110] Substrate solution (1.0mmol / L): Accurately weigh 16.7mg of HIPPURYL-L-ARGININE (Sigma), add 50mL of bioassay buffer to dissolve it, or dissolve it in equal proportions according to the actual weight weighed. After the configuration is completed, it is placed for 30 minutes to ensure that it is fully dissolved, and it is a 1.0mmol / L substrate solution. Ready to use. Preheat to 25°C+0.5°C.
[0111] Activity measurement: absorb 3.0mL of substrate solution, take a covered quartz cuvette with an optical path of 1cm, add 3.0mL of substrate solution preheated at 25°C, and immediately adjust to zero at a wavelength of 254nm. Precisely draw 10 μl of the recombinant CPB enzyme solution and add it, time it immediately and shake it well, and read the absorbance every 30 seconds for a total of 5 minutes. Measure three times and take the aver...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com