Protein, transmembrane nucleic acid unwinding nanopore, construction method and application thereof
A protein and DNA helicase technology, applied in the fields of application, nanotechnology, nanotechnology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
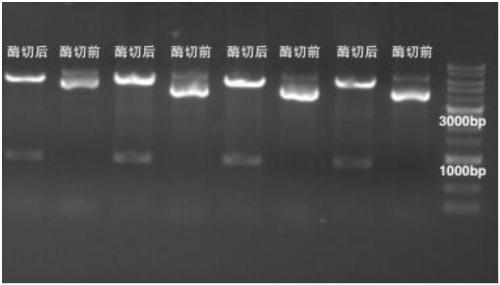
[0174] Example 1 E1 protein and its mutant recombinant vector construction and expression purification:
[0175] Amino acid sequence (SEQ ID No.3) of truncation E1-1 (306-577):
[0176]LQTEKFDFGTMVQWAYDHKYAEESKIAYEYALAAGSDSNARAFLATNSQAKHVKDCATMVRHYLRAETQALSMPAYIKARCKLATGEGSWKSILTFFNYQNIELITFINALKLWLKGIPKKNCLAFIGPPNTGKSMLCNSLIHFLGGSVLSFANHKSHFWLASLADTRAALVDDATHACWRYFDTYLRNALDGYPVSIDRKHKAAVQIKAPPLLVTSNIDVQAEDRYLYLHSRVQTFRFEQPCTDESGEQPFNITDADWKSFFVRLWGRLDL。
[0177] The coding gene sequence (SEQ ID No.4) of the truncated body E1-1 (306-577):
[0178] Ttgcagaccgagaaattcgacttcggaactatggtgcaatgggcctatgatcacaaatatgctgaggagtctaaaatagcctatgaatatgctttggctgcaggatctgatagcaatgcacgggcttttttagcaactaacagccaagctaagcatgtgaaggactgtgcaactatggtaagacactatctaagagctgaaacacaagcattaagcatgcctgcatatattaaagctaggtgcaagctggcaactggggaaggaagctggaagtctatcctaactttttttaactatcagaatattgaattaattacctttattaatgctttaaagctctggctaaaaggaattccaaaaaaaaactgtttagcatttattggccctcc aaacacaggcaagtctatgctctgcaactcattaattcattttttgg...
Embodiment 2
[0215] Embodiment 2. Fluorescent labeling and membrane fusion experiments of protein truncated bodies and mutants thereof:
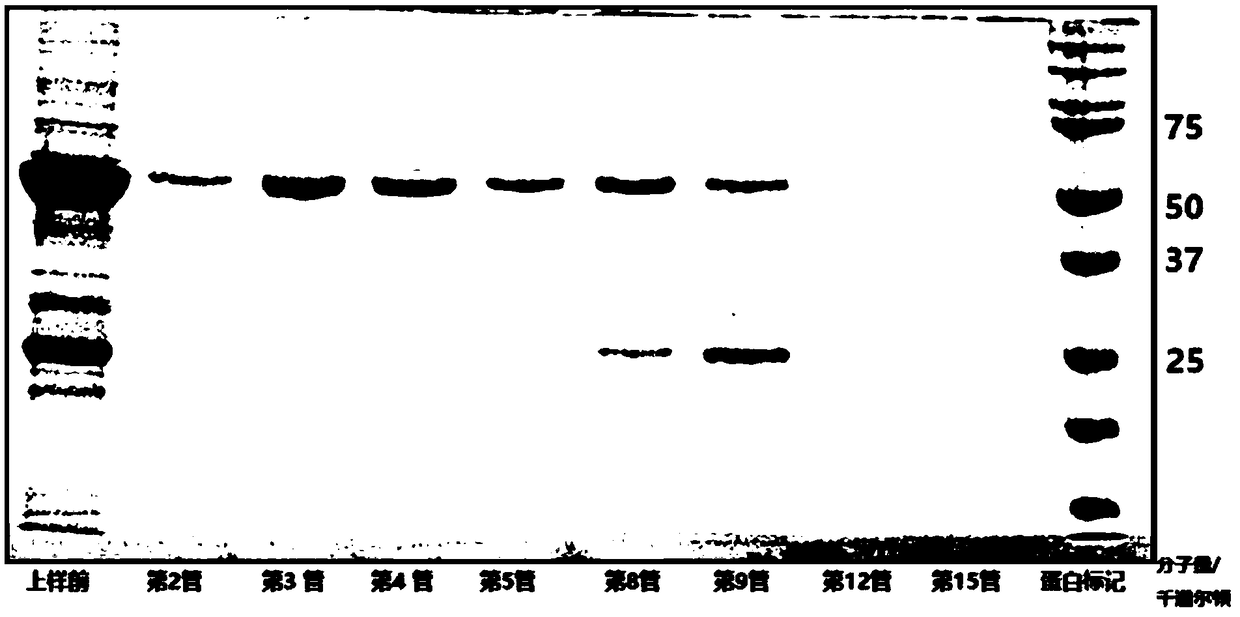
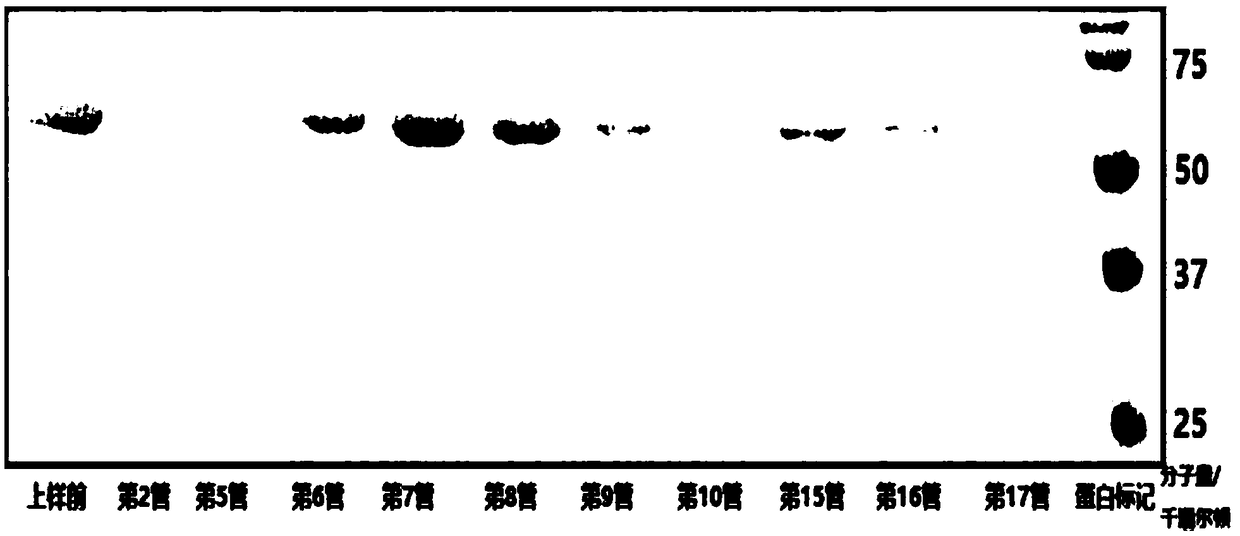
[0216] Fluorescence-labeled truncated E1-2 (306-605) protein: the material used was Fluorine-labeled Fluorescein Isothiocyanate (FITC) Conjugate Kit (Sigma, St. Louis, Missouri). According to the description of the kit, excess FITC was removed by column chromatography, and FITC-linked E1 was eluted with protein buffer (20 mM tris-HCl (Ph7.3), 200 mM NaCl). The FITC-labeled E1 (306-605) was verified by SDS-PAGE (figure omitted, the position of FITC-labeled E1-2 on SDS-PAGE is slightly higher than that of unlabeled E1-2).
[0217]Preparation of small vesicles (SUVs) containing truncated E1-2 (306-605): To prepare small vesicles containing E1 protein with a size of about 0.1 μm, 1 mg / ml DOPC was added to a vial, and nitrogen gas was used to After drying the chloroform, add an aqueous solution containing E1 protein (concentration is about 0.5 mg / ml), rehydr...
Embodiment 3 E1
[0222] Embodiment 3, E1 protein and its mutant electrophysiological experiment:
[0223] Electrophysiological measurement: the instrument is HEKA EPC-10USB, which integrates the amplifier and digital-to-analog conversion part. The two electrodes are the pressure (voltage clamp mode) electrode and the reference electrode, which are a pair of silver / silver chloride electrodes. . It is used to measure the transmembrane current across both sides of the phospholipid bilayer membrane. All sampling frequencies are at 2kHz, and the low-pass filtering frequency is 1kHz. Data collection was done by Patch-master software, and data analysis was done by Clamfit.
[0224] Construction of lipid bilayer: use horizontal standard lipid bilayer builder and vertical standard lipid bilayer box (BCH-13A, purchased from Warner.No.64-0451. Customized 50μm CUPs) to construct plane Lipid bilayer. The builder and the bilayer box were divided into cis-type by using a thin polytetrafluoroethylene mate...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com