Method for co-production of 5-keto-D-gluconic acid and ethyl (S)-4-chloro-3-hydroxybutyrate
A technology of ethyl hydroxybutyrate and gluconic acid, which is applied in the field of biocatalysis, can solve the problems of difficult to achieve complete conversion of gluconic acid, low oxygen concentration of the substrate, low binding efficiency, etc., and achieve green reaction, high selectivity, and process simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
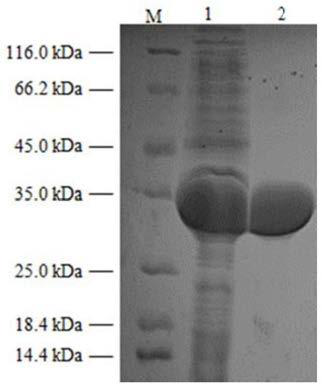
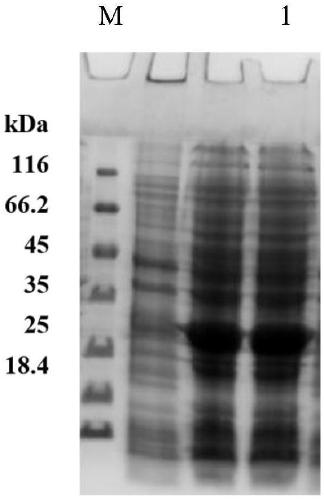
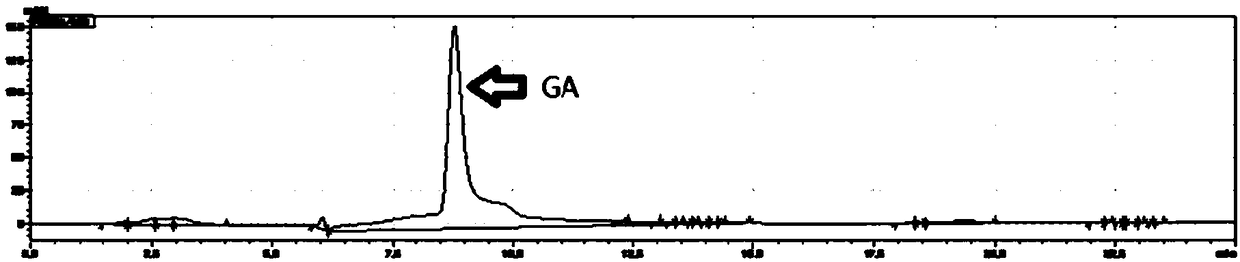
Image
Examples
Embodiment 1
[0027] Example 1: Derived from Gluconobacteroxydans The gluconate dehydrogenase gene ( gdh ) clone
[0028] Gluconobacteroxydans Purchased from China General Microorganism Culture Collection and Management Center (CGMCC 1.637). Gluconobacter oxydans culture adopts the medium formula (g / L): glucose 100, yeast extract 10, calcium carbonate 20, agar 15, completely dissolved in deionized water, adjusted to pH 6.8, constant volume, high pressure steam sterilization .
[0029] Gluconobacter oxydans was inoculated into LB liquid medium, cultured at 37°C to logarithmic growth phase, and the genome of Streptococcus thermophilus was extracted using DNAKit (TIANGEN, China). The primers used to construct the expression vector are added with restriction enzyme sites, and the primer sequences are as follows:
[0030]Upstream primers (gluconate dehydrogenase-sense with EcoRI) are:
[0031] 5'-CCGGAATTCATGAGTACCTCTCTTTTTG-3'
[0032] Downstream primers (gluconate dehydrogenase-anti ...
Embodiment 2
[0035] Example 2: Recombinant expression vector pET-28a- gdh build
[0036] Use EcoRI and HindIII to digest (purchased from Novagen Merck China) and the amplified target gene containing two restriction sites, respectively, and recover the double-digested target fragment and expression vector, respectively. The expression vector pET-28a and the target gene (the accession number in Genbank is NC_019396.1) were ligated overnight with T4-DNA ligase (purchased from TaKaRa company) to obtain the recombinant vector pET-28a- gdh ; Add 10 μL of the ligation product to E. coli BL21 competent cells, place on ice for 30 min, and heat shock at 42°C for 90 s. Place on ice for 2min. Add pre-warmed 0.45 mL of LB medium. 220rpm, 37°C, 1h. Add 200 μL of bacterial solution to a plate containing 30 μg / mL kanamycin, and culture at 37°C for 12-16 hours overnight to obtain recombinant bacteria. E.coli BL21 (with pET-28a- gdh ).
Embodiment 3
[0037] Example 3: Carbonyl reductase gene derived from ScheffersomycesstipitisCBS6054 ( sou2 ) source and acquisition
[0038] This example provides a polynucleotide molecule encoding carbonyl reductase activity, the nucleotide molecule encoding Genbank accession number XP-001387287, and artificially synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com