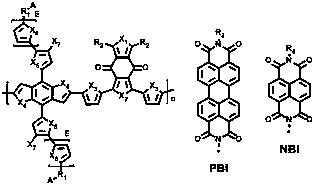
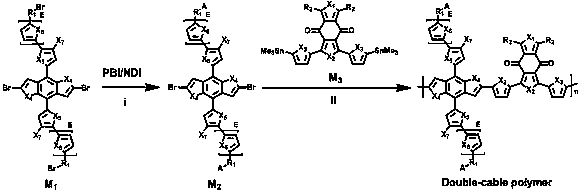
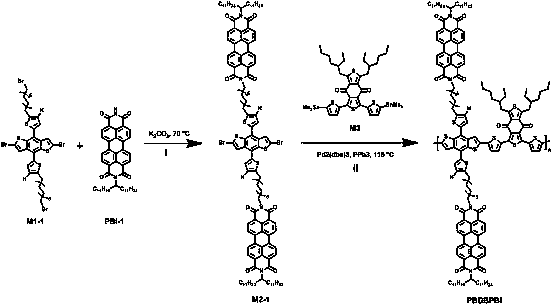
Preparation method and application of double-cable polymer
A polymer and dual-cable technology, applied in the fields of semiconductor/solid-state device manufacturing, photovoltaic power generation, electrical components, etc., can solve the problems of low photoelectric energy conversion efficiency, and achieve a simple and effective synthesis method, improve stability, and simplify the preparation process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of benzodithiophenedione-benzodithiophene type double cable polymer PBDBPBI:
[0048] The synthetic reaction formula of double-cable polymer PBDBPBI is as follows image 3 shown.
[0049] Concrete synthetic steps are:
[0050] (1) In a 100mL reaction flask, dissolve dialkyl bromide-substituted benzodithiophene M1-1 (225mg, 0.22mmol), PBI-1 (350mg, 0.49mmol) into 30mL DMF, and add Potassium carbonate (93 mg, 0.67 mmol). Heat to 75 degrees Celsius, react for 24 hours, after cooling, add 100 mL each of chloroform and deionized water. The liquid was separated, the organic phase was washed with brine, dried and rotary evaporated to obtain the crude product. The crude product was separated through a silica gel column, eluent dichloromethane:petroleum ether=4:1 (V / V), to obtain pure M2-1 with a yield of about 50%. 1 H NMR (400MHz, CDCl3): δ (ppm) 8.60 (m, 8H), 8.53 (m, 8H), 7.52 (s, 2H), 7.16 (s, 2H), 6.87 (s, 2H), 5.18 (t ,2H),4.18(t,4H),2.89(t,4H),2.26(m,4H),...
Embodiment 2
[0055] Synthesis of benzodithiophenedione-benzodithiophene type double cable polymer PBDBPBI-Cl:
[0056] The synthetic reaction formula of double-cable polymer PBDBPBI-Cl is as follows Figure 4 shown.
[0057] Concrete synthetic steps are consistent with embodiment 1.
[0058] The only difference is that benzodithiophene M1-2 substituted with chlorinated dialkyl bromide is used as the starting material, and the obtained intermediate M2-2 1 H NMR picture as Figure 9 as shown, 13 C NMR picture as Figure 10 shown. PBDBPBI-Cl 1 H-NMR such as Figure 11 shown.
[0059] The ultraviolet-visible absorption spectrum (UV-vis) of PBDBPBI-Cl is as Figure 13 Shown; The J-V curve of the single-component organic solar cell device of PBDBPBI-Cl is as Figure 14 Shown; The external quantum efficiency (EQE) of the single-component organic solar cell device of PBDBPBI-Cl is as Figure 15 shown.
Embodiment 3
[0061] Synthesis of benzodithiophenedione-benzodithiophene double cable polymer PBDBNDI:
[0062] The synthetic reaction formula of double-cable polymer PBDBNDI is as follows Figure 5 shown.
[0063] Concrete synthetic steps are:
[0064] (1) In a 100mL reaction flask, dissolve the dialkyl bromide-substituted benzodithiophene M1-1 (98mg, 0.091mmol), NDI-1 (124.5mg, 0.23mmol) in 15mL DMF, and wait for the dissolution equilibrium Potassium carbonate (38 mg, 0.28 mmol) was added.
[0065] Heat to 75 degrees Celsius, react for 12 hours, and suction filter while hot, wash the obtained solid several times with hot water, and then wash 50 mL with hot ethanol. After vacuum drying, pure M2-3 was obtained with a yield of about 87%. 1 H NMR (400MHz, CDCl 3 ): δ(ppm)8.74(s,8H),7.53(s,2H),7.14(s,2H),4.20(d,4H),4.11(d,4H),2.87(t,4H),1.98( s,2H),1.30(m,92H),0.84(m,12H).M2-3 1 H NMR as Figure 12 shown.
[0066] (2) M2-3 (39.27 mg, 19.6 μmol), M3-1 (18.30 mg, 19.6 μmol) were dissolv...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com