Sugar-polyethylene glycol-DSPE (1,2-distearoyl-sn-glycero-3-phosphoethanolamine) conjugated compound and preparation method and application thereof
A technology for coupling compounds and polyethylene glycol, applied in the field of medicine, can solve the problems of synthetic process reports, complex synthetic processes, difficult post-processing, etc., and achieves the effects of high yield, simple operation, and easy control of reaction conditions.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
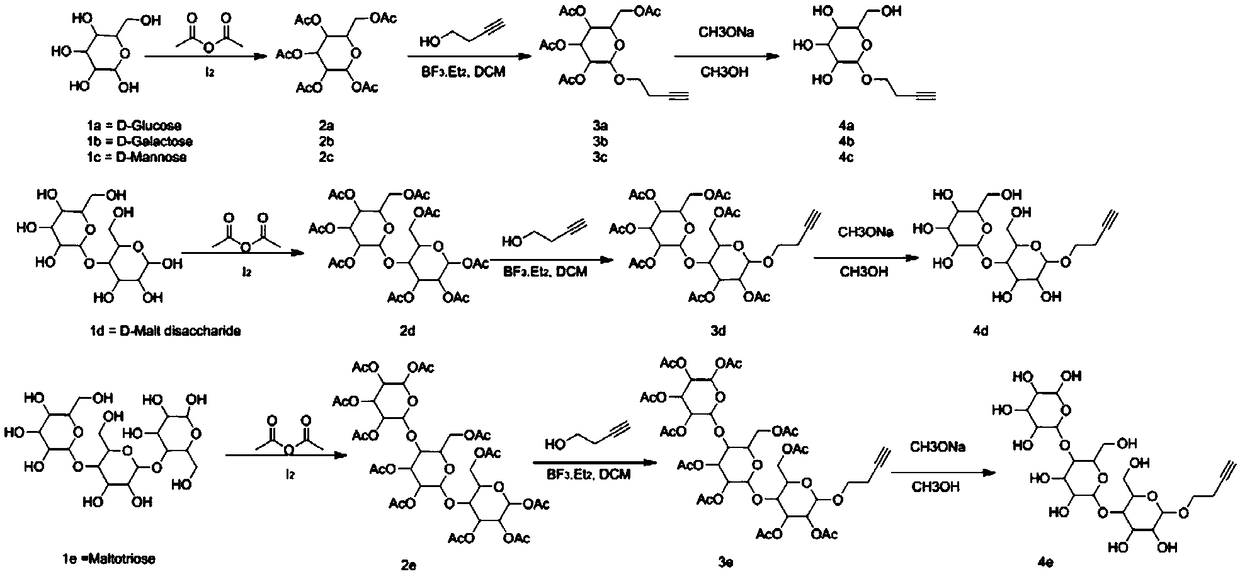
[0091] The synthesis of embodiment 1 compound 2a or 2b or 2c or 2d or 2e
[0092]
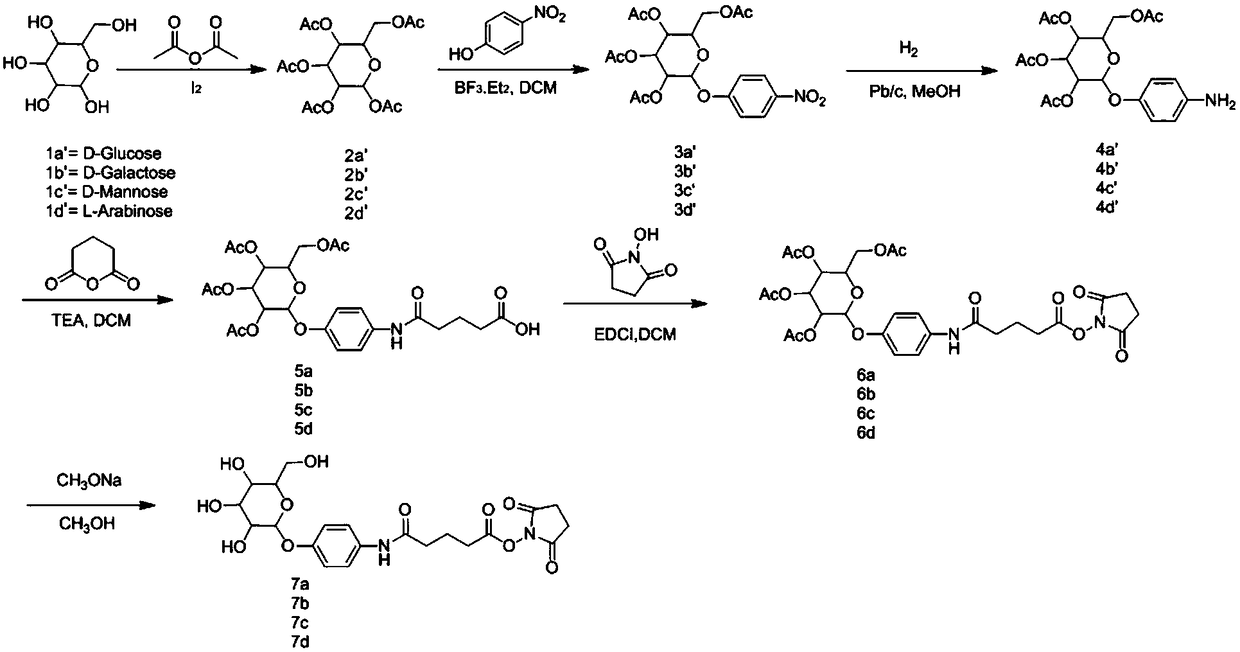
[0093] Operation process: Add 1g of glucose (galactose, mannose, maltobiose, maltotriose), 5ml of acetic anhydride, 7ml of pyridine, and 10mg of iodine tablets into a three-necked flask in sequence, and carry out acetylation reaction at 0°C, and 25°C React overnight (there is heat dissipation in the reaction system when just added). The next day, add 8.4g NaHCO 3 Aqueous solution (10ml H 2 O) in 30ml EA, stir for 2h, bubbles are generated, use NaHCO 3 Wash once with aqueous solution, then wash twice with 2M HCl, and spin dry to obtain compound 2a. Results: 4.1 g of a colorless and transparent oily product was obtained, with a yield of 94.7%. 2b, 2c, 2d, 2e were synthesized under the same conditions as 2a.
[0094] NMR analysis of compound 2a:
[0095] H NMR (500MHz; Chloroform-d): δ6.29(d, J=3.2Hz, 1H), 5.67(d, J=7.7Hz, 0.4H), 5.44-5.35(dd, J=10.2and 10.0Hz, 1H),5.29-5.21(m,0.4H),5.17...
Embodiment 2
[0096]The synthesis of embodiment 2 compound 3a or 3b or 3c or 3d or 3e
[0097]
[0098] Operation process: Add compound 2a (2g, 5.1mmol), 3-butyn-1-ol (718mg, 10.2mmol), dichloromethane (20ml) into a 100ml three-necked flask in turn, protect with nitrogen, cool down to 4°C in an ice bath, A solution of boron trifluoride in ether (1.25ml, 10.2mmol) was added dropwise, and reacted overnight at room temperature. On the second day, saturated aqueous sodium bicarbonate solution (30ml) was added to quench the reaction, dichloromethane (50ml) was added to extract, dried over anhydrous sodium sulfate, filtered, and spin-dried to obtain 2.01g of a yellow semi-oil and semi-solid product, namely compound 2c, collected The rate is 98.0%. 3b, 3c, 3d, 3e were synthesized under the same conditions as 3a.
Embodiment 3
[0099] The synthesis of embodiment 3 compound 4a or 4b or 4c or 4d or 4e
[0100]
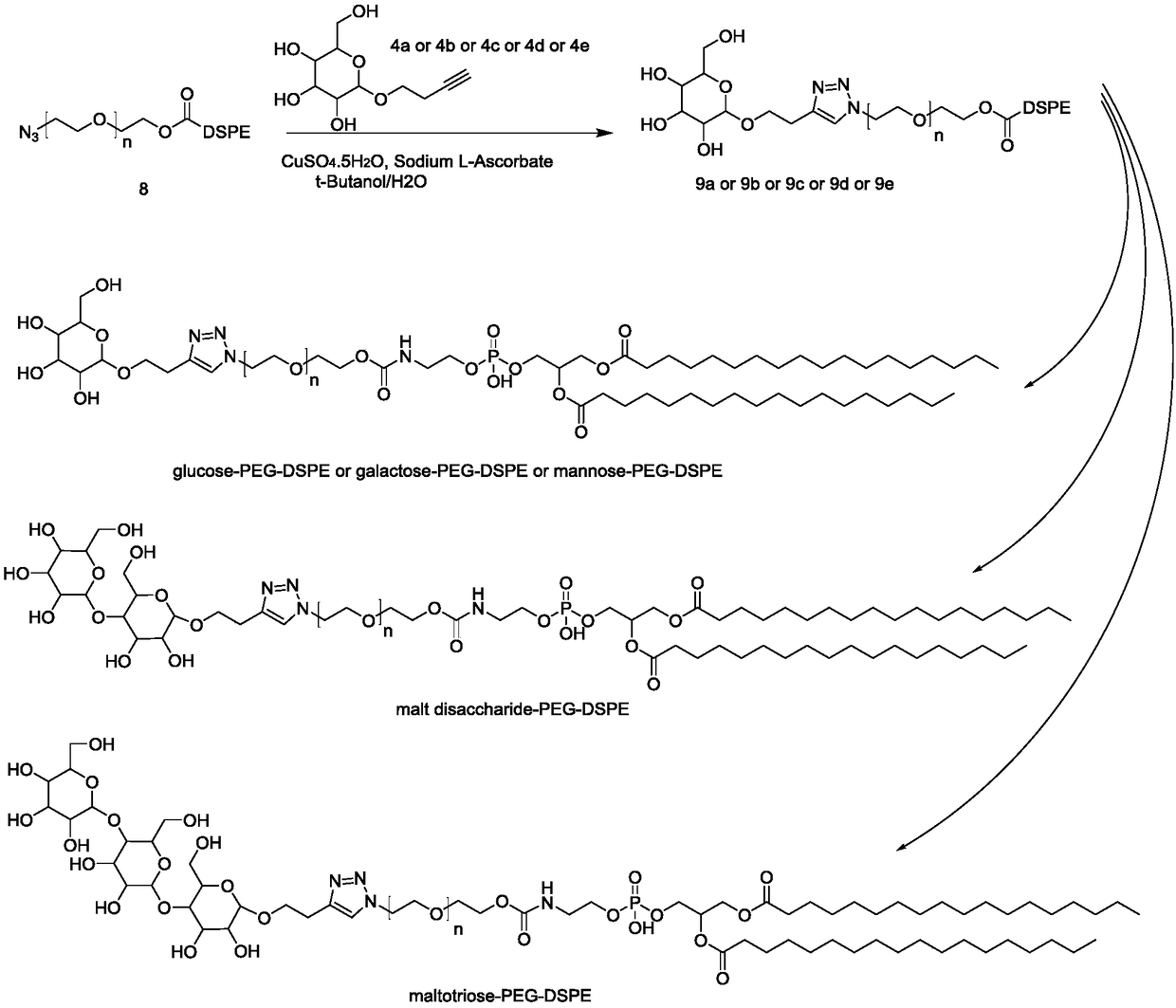
[0101] Operation process: Compound 3a (2.01g, 5mmol) and methanol (20ml) were sequentially added into a 100ml three-neck flask, protected by nitrogen, then sodium methoxide (1.35g, 25mmol) was added, and stirred overnight at room temperature. On the second day, add glacial acetic acid (1.8ml, 30mmol) and stir for 10 minutes, spin dry, add deionized water (5ml) to dissolve, purify with ion exchange resin, and spin dry to obtain 1.1g of off-white semi-oil and semi-solid product, that is, compound 4a , yield 94.3%. 4b, 4c, 4d, 4e were synthesized under the same conditions as 4a.
[0102] Compound 4a NMR analysis: 1H NMR (500MHz, Methanol-d4) δ 4.30 (d, J = 7.8Hz, 1H), 3.99-3.92 (m, 1H), 3.86 (dd, J = 11.9, 1.7Hz, 1H) ,3.72–3.63(m,2H),3.35–3.24(m,3H),3.17(dd,J=9.1,7.8Hz,1H),2.54-2.48(m,2H),2.27(t,J=2.7Hz ,1H).
[0103] Compound 4b NMR analysis: 1H NMR (500MHz, Methanol-d4) δ4.25(d, J=7.5Hz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
| fluorescence | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com