Preparation method and application of recombinant vaccine host protein antibody
A host protein and vaccine technology, which is applied to the preparation method of peptides, immunoglobulins, antiviral immunoglobulins, etc., can solve the problems of low actual measured value, inability to fully reflect HCP residues, and reduced sensitivity, and achieve detection Improve sensitivity, save test operation time and cost, and have good applicability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0049] Embodiment 1 Construction of Hansenula empty vector expression strain
[0050] The Hansenula expression vector PMV-05 (CN 201210592813.5) was transformed into Hansenula uracil-deficient host strain AU-0501 by electroporation (the construction method of Hansenula expression vector and Hansenula uracil-deficient host strain can be See CN 201210592813.5), the recombinant Hansenula empty vector expression strain was obtained through stable culture and screening.
Embodiment 2
[0051] The preparation of embodiment 2 Hansenula HCP
[0052] 1. Fermentation culture of recombinant Hansenula empty vector expression strain
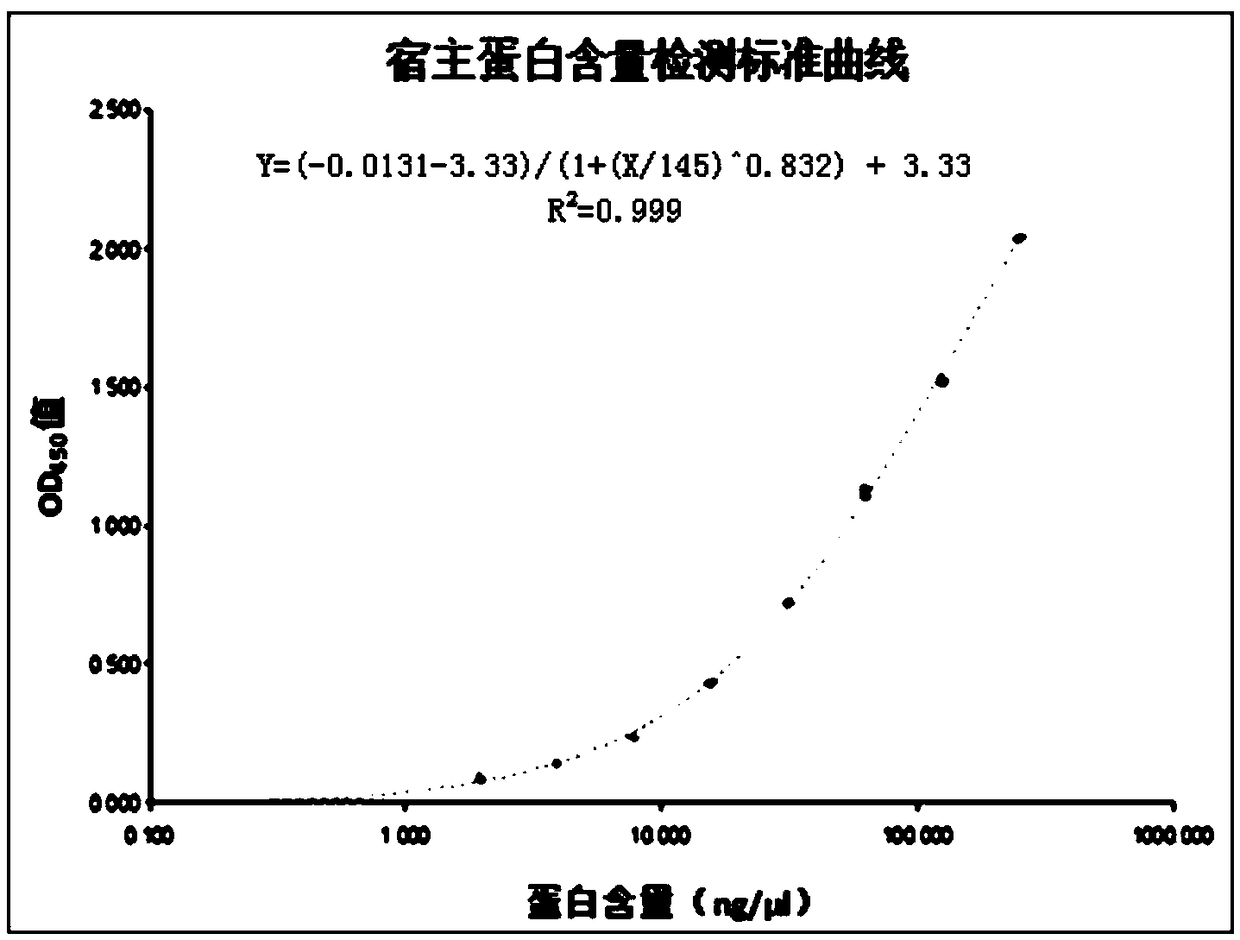
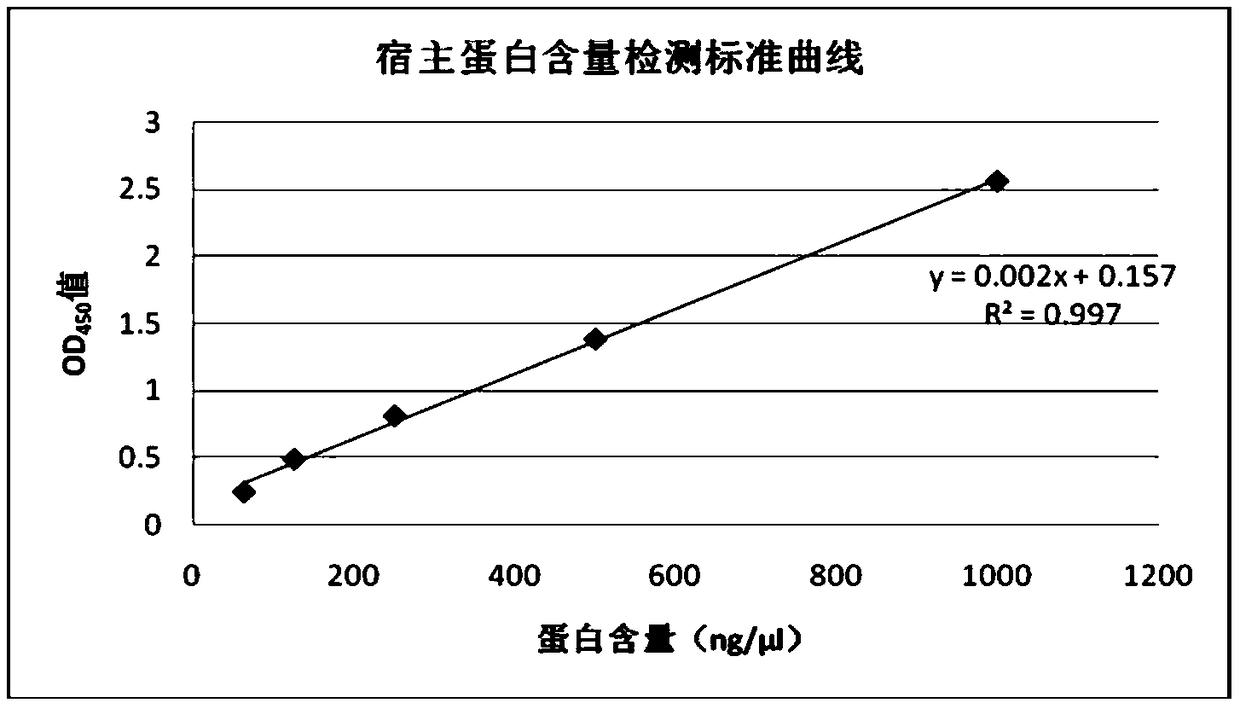
[0053] The recombinant Hansenula empty vector expression strain was inoculated into 100ml primary seed medium (yeast extract 6.7g / L, ammonium sulfate 5g / L, glucose 20g / L), and cultured at 33°C and 200rpm shaking table for 20~ 24 hours. Then inoculate the whole amount in 1000ml secondary seed medium (yeast extract 6.7g / L, ammonium sulfate 5g / L, glycerol 20g / L), inoculate at 33°C, 200rpm shaker for 20-24 hours and then inoculate to 30L In the fermentation tank, 12L fermentation medium (glycerol 23.333g / L, ammonium dihydrogen phosphate 11.667g / L, 3.333g / L potassium chloride, calcium chloride 2.500g / L, sodium chloride 0.333g / L , magnesium sulfate 2.500g / L, sodium edetate 0.167g / L), ammonia water to adjust the pH value of the fermentation broth to maintain at 5.0, the fermentation temperature is 30°C, the speed is controlled at 350-750rpm...
Embodiment 3
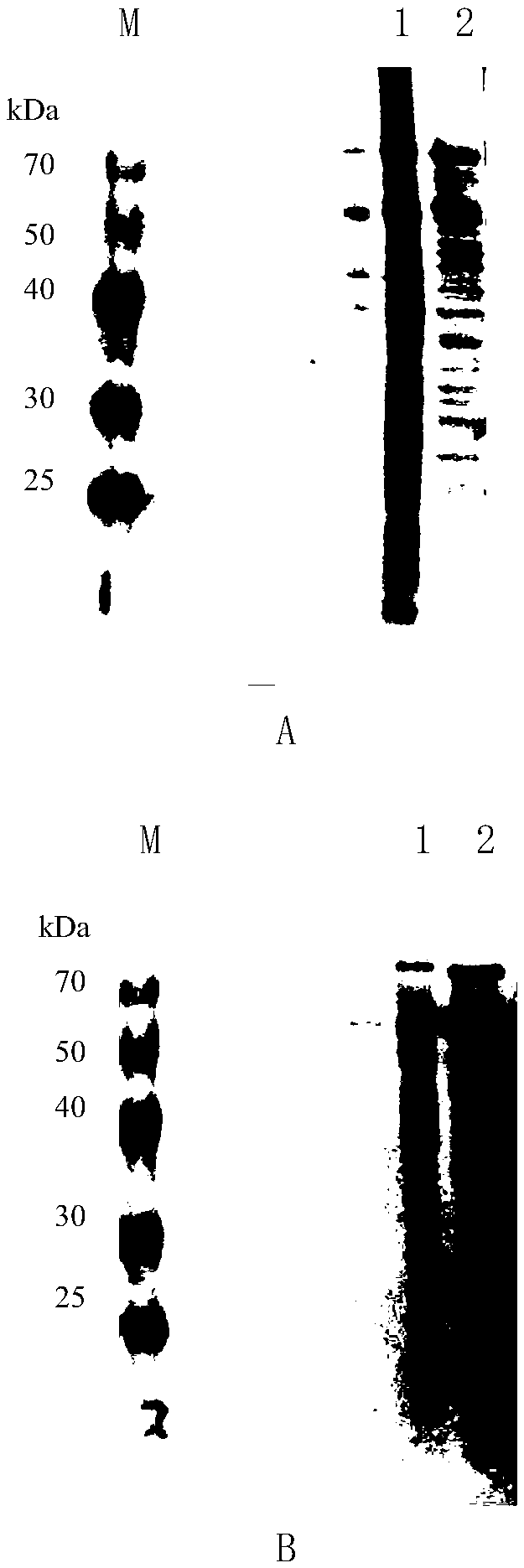
[0064] Example 3 Preparation of Hansenula HCP Antibody
[0065] 1 animal immunity
[0066] 1.1 Rabbit Immunization
[0067] First immunization: take 6ml of host protein diluted to 1mg / ml, add 6ml of Freund's complete adjuvant (FCA) for emulsification, take one drop until the water surface does not disperse, and the emulsification is complete. Use a 2ml syringe to draw 2ml of Freund's complete adjuvant (FCA) emulsion (containing 1mg of host protein), and inject 0.2mL subcutaneously at multiple points on the neck and back.
[0068] The second immunization: After an interval of 10-14 days, the second immunization is carried out. Take 6ml of host protein diluted to 0.5mg / mL, add 6ml of Freund's incomplete adjuvant (FIA) for emulsification, take one drop until the water surface does not disperse, and the emulsification is complete. Use a 2ml syringe to draw 2ml of Freund's incomplete adjuvant (FIA) emulsion (containing 0.5mg of host protein), and inject 0.2mL at multiple points ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com