A kind of sulfur dioxide derivative fluorescent probe and its preparation method and application
A technology for fluorescent probes and sulfur dioxide, applied in chemical instruments and methods, fluorescence/phosphorescence, organic chemistry, etc., can solve problems such as impact, poor selectivity of probes, hindering the development of sulfur dioxide derivatives detection, etc., to reduce interference and damage , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] A kind of synthetic method of fluorescent probe, concrete steps are as follows:
[0041] (1) Synthesis of compound 1:
[0042]
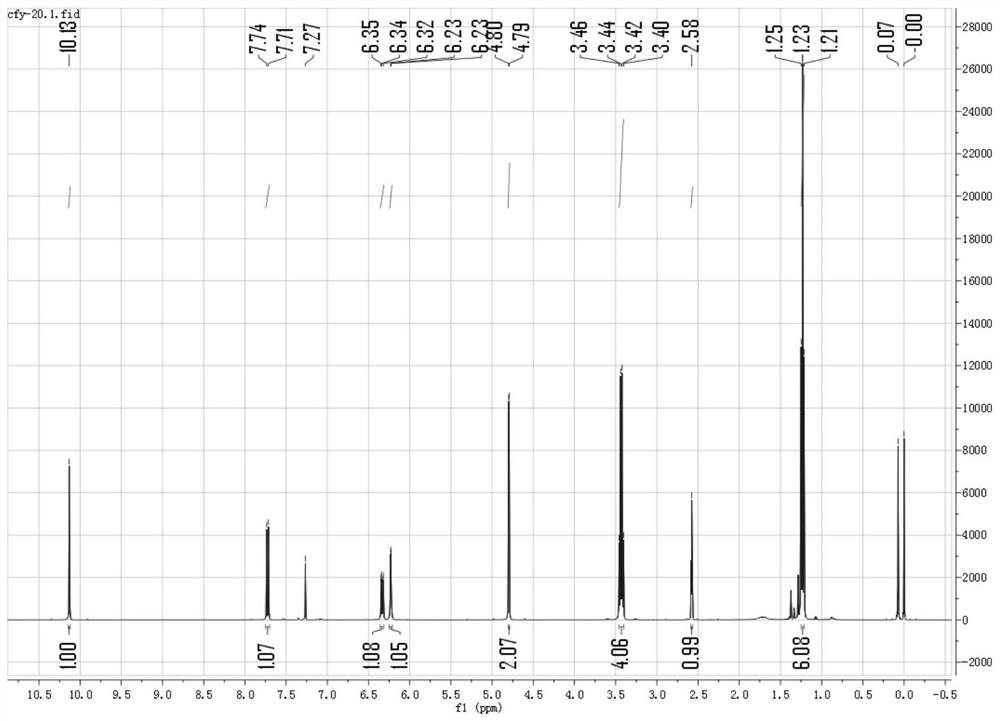
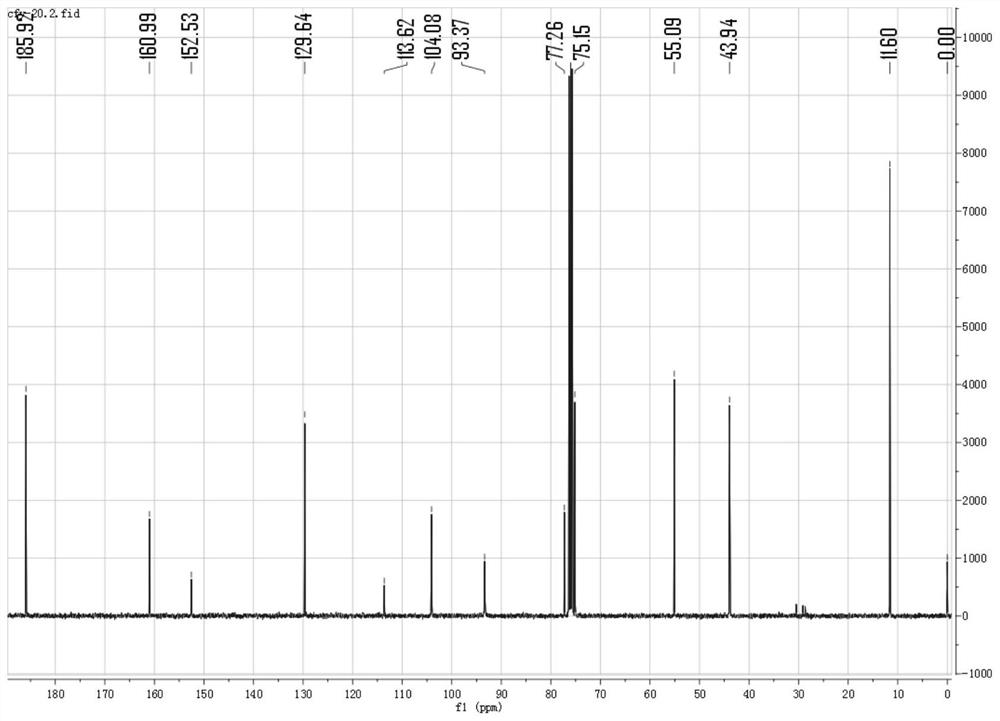
[0043] Get 960mg 4-(diethylamino) salicylaldehyde (molecular formula is: C 11 h 15 NO 2 ) (5mmol) and 700mg 3-bromopropyne (molecular formula is: C 3 h 3 Br) (5.8mmol) in the round bottom flask, add DMF to make it dissolve completely, then add 750mg potassium carbonate (molecular formula is: KCO 3 ), stirred under nitrogen protection and normal temperature for 10 hours, the reaction was completed, the reaction solution was washed three times with 50mL brine, and extracted with dichloromethane, and the solvent (dichloromethane) was removed by rotary evaporator vacuum distillation to obtain a crude product. Using dichloromethane and petroleum ether as the eluent, gradient elution was carried out at a volume ratio of 1:10 to 1:2, and purification was performed on a silica gel (200-300 mesh) chromatography column to obtain 806 mg of a whit...
Embodiment 2
[0051] Fluorescent probe Probe-SO 2 Applications:
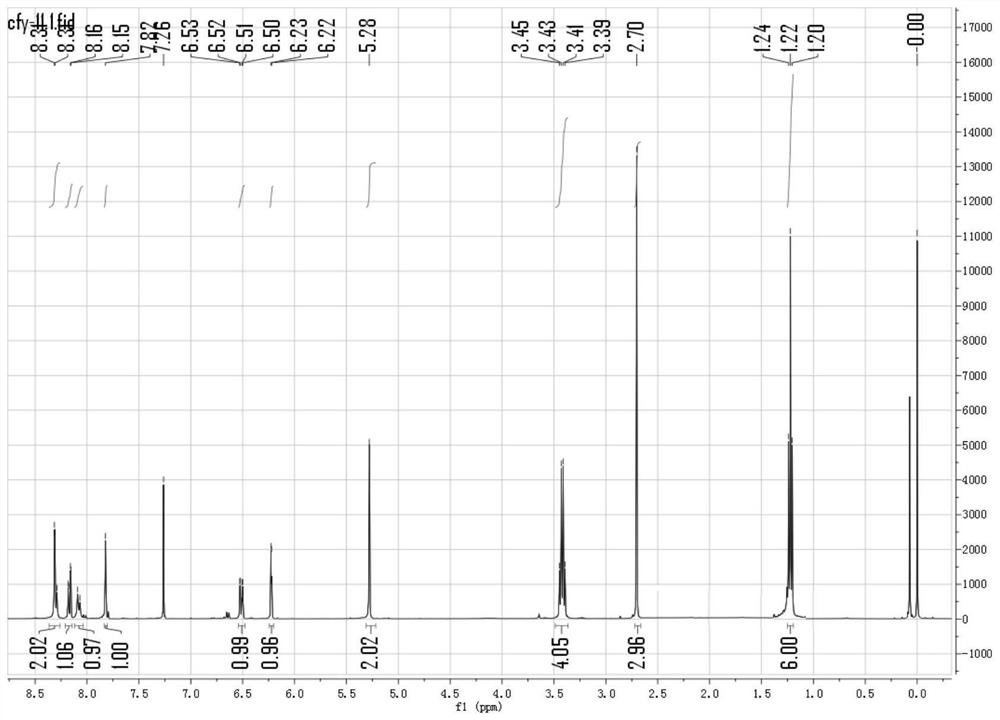
[0052] Dissolve the probe prepared in Example 1 in ethanol to make a probe mother liquor with a concentration of 1mmol / L, and dissolve 10.4mg (0.1mmol) of sodium bisulfite solid in 10mL of deionized water to obtain a concentration of 10mmol / L. Mother liquor of sulfur dioxide derivatives of L. Take 30 μ L from the probe mother liquor and add it to a 5mL centrifuge tube, add different equivalents (0-100eq) of sulfur dioxide derivative mother liquor (the equivalent refers to the molar number of sulfur dioxide derivatives in the sulfur dioxide derivative mother liquor relative to the probe mother liquor Multiples of the number of moles of the probe in the medium), diluted to 3 mL with different volumes of PBS aqueous solution (concentration 25 mmol / L, pH 7.4), and configured as a test solution with a probe concentration of 10 μmol / L containing 1% ethanol. Test the fluorescence spectrum change (excitation wavelength is 415nm) of...
Embodiment 3
[0055] Fluorescent probe Probe-SO 2 Applications:
[0056] Take 30 μL from the fluorescent probe mother solution in Example 2 and add it to a 5mL centrifuge tube, add 30 μL of sulfur dioxide derivative mother solution with a concentration of 100mmol / L, and then dilute to 3mL with 3ml PBS aqueous solution (concentration 25mmol / L, pH 7.4) , prepared as a test solution with a probe concentration of 10 μmol / L and containing 1% ethanol. Using the excitation wavelength of 525nm, test its fluorescence spectrum changing with time. Depend on Figure 8 It can be seen that as time increases, the fluorescence intensity at 525 nm gradually increases and reaches the maximum within 5 seconds.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com