Unsymmetric bifunctional carborane derivatives, and preparation method and application thereof
A bifunctional, carborane technology, applied in chemical instruments and methods, drug combinations, pharmaceutical formulations, etc., can solve the problems of low concentration and limit applications, and achieve the effect of improving high temperature resistance, thermal stability, and great application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
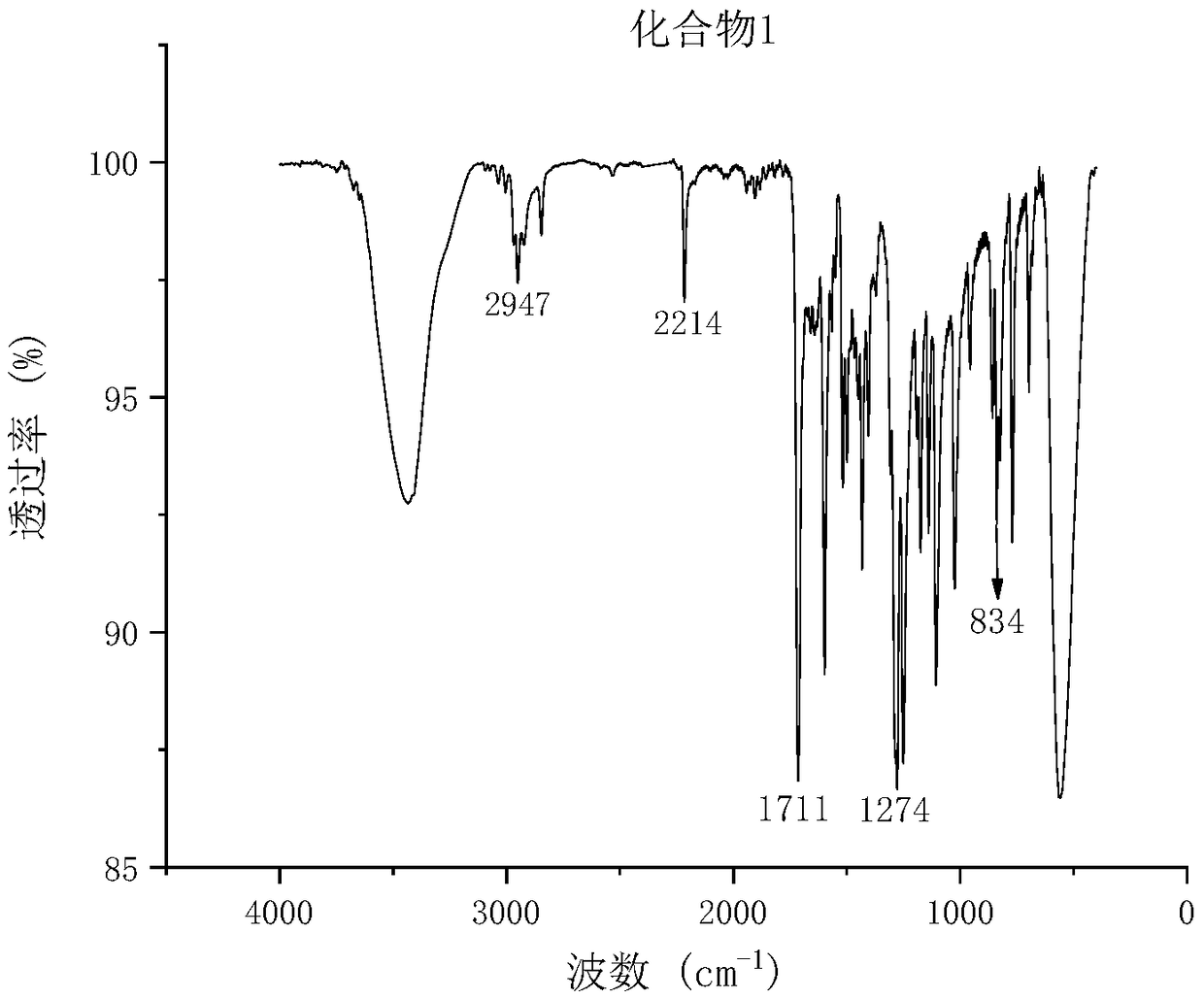
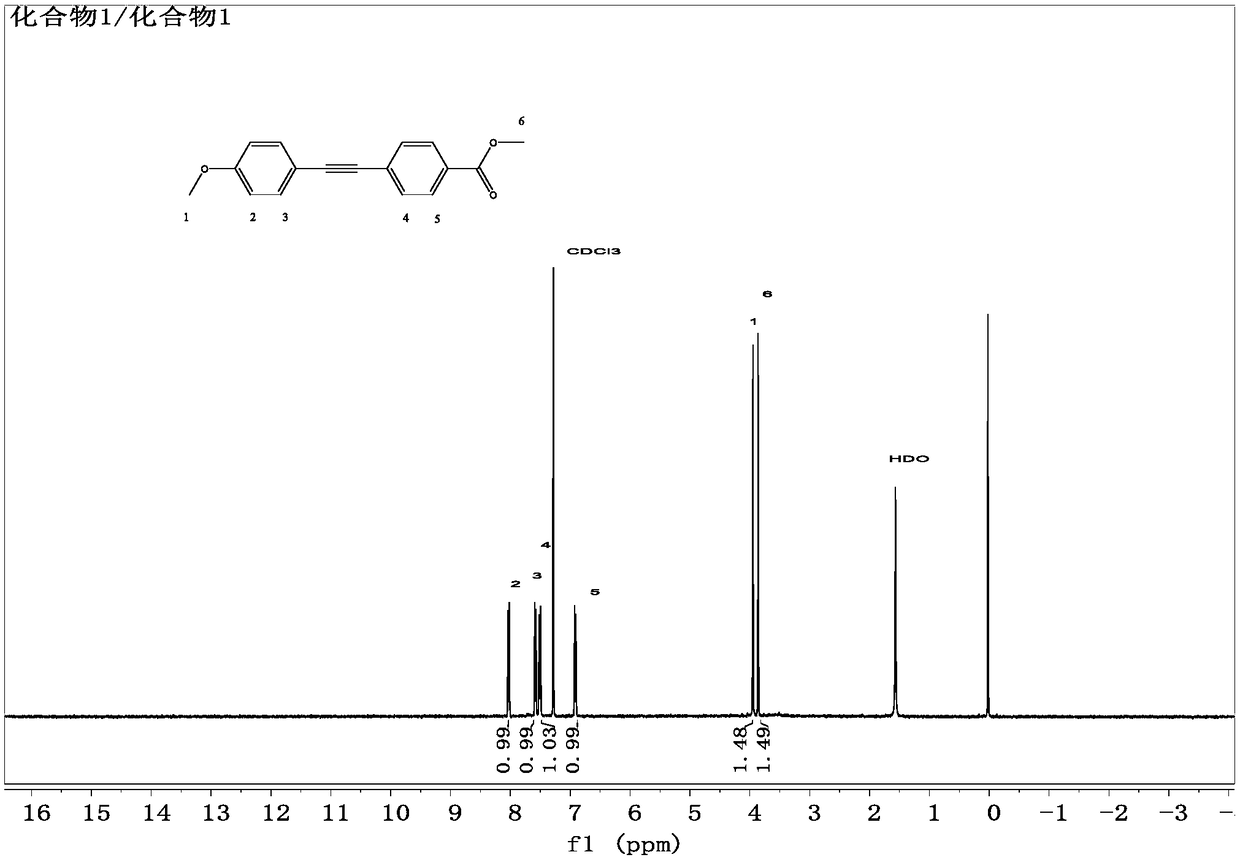
[0054] The synthesis of embodiment 1 compound 1
[0055] Add 2.32g (10mmol) of methyl 4-iodobenzoate, 120ml of dewatered triethylamine, and 15ml of dehydrated THF into a three-necked flask equipped with stirring, connect the drying device and oxygen removal device, and turn on the magnetic stirring. Pass high-purity nitrogen for about 1 hour, add tetrakistriphenylphosphine palladium 115mg (0.1mmol), cuprous iodide 38mg (0.2mmol), heat up to 50°C, keep warm for 30min, and take 1.45g (11mmol) of 4-methoxy with a syringe Phenylacetylene was quickly injected, and the reaction was monitored by TLC (solid precipitation occurred). The product was filtered, the mother liquor was spin-dried, the solid obtained twice was dissolved with 50ml of dichloromethane (DCM), the organic phase was washed with 1N hydrochloric acid, dried by adding anhydrous magnesium sulfate for 3h, magnesium sulfate was removed, and the solvent was spin-dried to obtain The white solid was vacuum-dried at 40° C. to...
Embodiment 3
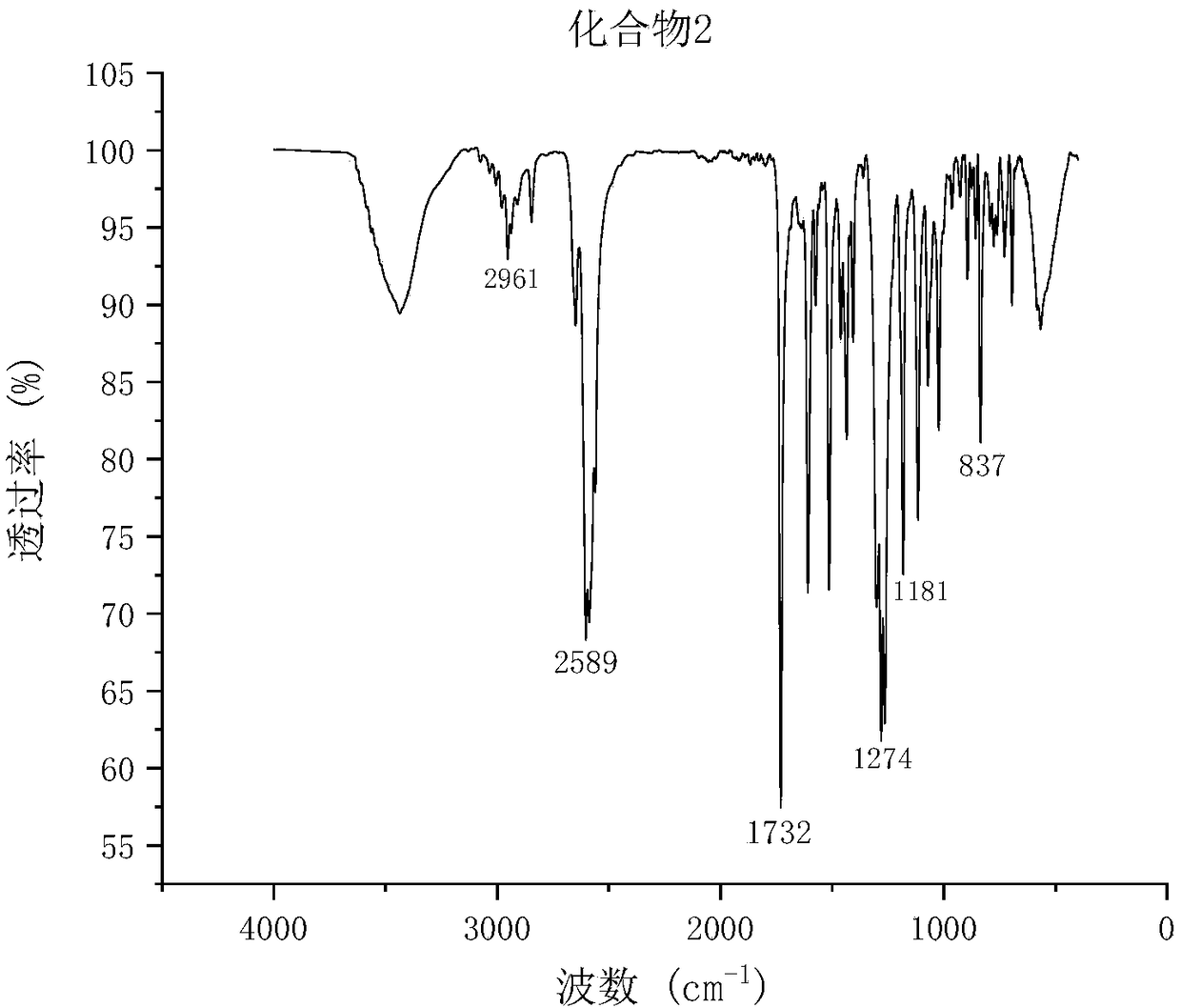
[0061] The synthesis of embodiment 3 compound 2
[0062] Add 4.7g of decaborane aniline complex (15mmol) and 100ml of dehydrated toluene to a three-necked flask equipped with a stirring and condensing reflux device, pass nitrogen gas for 30min, turn on magnetic stirring, and add 2.66g (10mmol) of compound 1 , react at room temperature for 1 h, raise the temperature to 110° C., remove the nitrogen, and monitor the completion of the reaction by TLC. The product was cooled to room temperature, and the filtrate (orange-red) was taken by suction filtration, the organic phase was washed with 1N hydrochloric acid, and then washed with distilled water until PH = 7, and anhydrous magnesium sulfate was added to the organic phase after liquid separation. Toluene gave an orange-red viscous liquid, which was recrystallized from ice ethanol and filtered to obtain a white powder, which was dried in vacuum to obtain compound 2 (1.73 g, yield 46.01%). mp: 103°C.
[0063] FT-IR (KBr, cm -1 )...
Embodiment 5
[0066] The synthesis of embodiment 5 compound 3
[0067] Add 3.76g (10mmol) of compound 2 into a three-necked flask equipped with stirring, add 100ml sodium hydroxide solution (0.5mol / L), 60ml tetrahydrofuran, stir, heat up to 65°C, and monitor the reaction by TLC. After the reaction was finished, the pH was adjusted to <2 with concentrated hydrochloric acid, stirred for 30 min, THF was removed by rotary evaporation, recrystallized with distilled water after suction filtration to obtain a white solid, and compound 3 (3.12 g, yield 86%) was obtained by vacuum drying.
[0068] FT-IR (KBr, cm -1 ): 3061-2833 broad peak (carboxyl stretching vibration absorption peak), 2640 and 2592 (-B-H stretching vibration), 1705 (C=O stretching vibration of ARCOOH dyad), 1418 (bending vibration of carboxyl-OH), 1260 (C=O stretching vibration of ether), 1181 and 1081 (characteristic peaks of boron cage skeleton), 940 (bending vibration of carboxyl-OH), 834 (stretching vibration of para-substitu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com