Method for catalytic synthesis of piperazine and triethylenediamine from ethylenediamine under solvent-free condition
A technology for the catalytic synthesis of piperazine and triethylenediamine catalyzed by ethylenediamine, which is applied in the direction of organic chemistry, can solve the problems of increased solvent recovery load, difficulty in azeotropic distillation separation, increased production costs, etc., and achieves the reduction of by-product yield, The separation process is simplified and the production cost is reduced
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
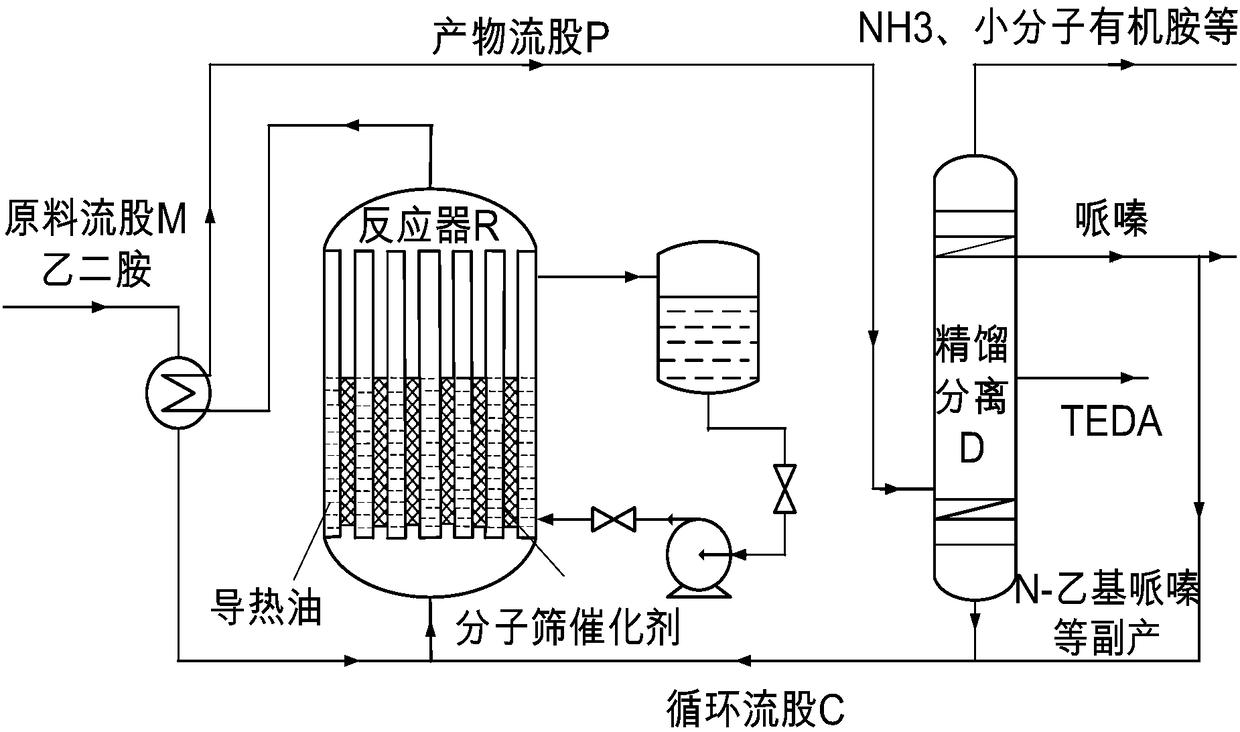
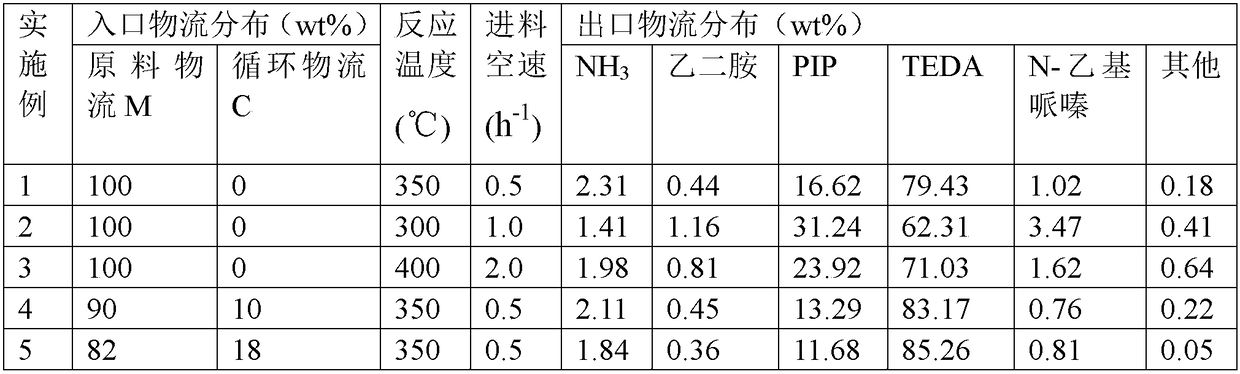
[0030] The catalyst used in this example is the hierarchically porous ZSM-5 molecular sieve disclosed in CN105712379A mentioned above, ion-exchanged with ammonium acetate and calcined in air at 500°C to obtain a hierarchically porous H-ZSM-5 molecular sieve catalyst. refer to figure 1 , the reaction process is: the feed mass space velocity of the raw material stream M is 0.5h -1 , after being preheated to 350°C, it enters the tube side of the reactor R with a tube-shell structure, and fills it with a multi-stage porous H-ZSM-5 molecular sieve catalyst. After the raw material stream M undergoes a contact reaction, the The product stream P obtained at the top extraction outlet was sampled for gas chromatography analysis, and the results obtained are shown in Table 1.
[0031] The product stream P from the reactor directly enters the rectification tower (it has a tower top and a bottom extraction outlet and an upper zone and a lower zone sideline extraction outlet; the number of...
Embodiment 2
[0033] The catalyst used in this embodiment is the mesoporous and microporous mordenite molecular sieve catalyst disclosed in CN106032281A mentioned above as the multi-level porous mordenite molecular sieve catalyst. refer to figure 1 , the reaction process is: the feed mass space velocity of the raw material stream M is 1.0h -1 , after being preheated to 300°C, it enters the tube side of the reactor R with a tube-shell structure, where it is filled with a multi-stage porous mordenite molecular sieve catalyst. The obtained product stream P was sampled and analyzed by gas chromatography, and the results obtained are shown in Table 1.
[0034] The product stream P from the reactor directly enters the rectification tower (it has a tower top and a tower bottom outlet and an upper zone and a lower zone sideline outlet; the number of theoretical plates is 30, the temperature of the tower bottom is 185 ° C, and the temperature of the tower top is 110 DEG C), through the rectifying ...
Embodiment 3
[0036] This embodiment uses the Beta molecular sieve with the hierarchical pore structure disclosed in CN104418348A mentioned above to undergo the ammonium acetate ion exchange and the hierarchically porous H-Beta molecular sieve obtained after roasting in air at 500°C as the hierarchically porous H-Beta molecular sieve catalyst . refer to figure 1 , the reaction process is: the feed mass space velocity of the raw material stream M is 2.0h -1 , after being preheated to 400°C, it enters the tube side of the tube-shell reactor R, where it is filled with a multi-stage porous H-Beta molecular sieve catalyst. The product stream P obtained at the outlet was sampled and analyzed by gas chromatography, and the results obtained are shown in Table 1.
[0037] The product stream P from the reactor directly enters the rectification tower (it has a tower top and a tower bottom outlet and an upper zone and a lower zone sideline outlet; the number of theoretical plates is 70, the temperatu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com