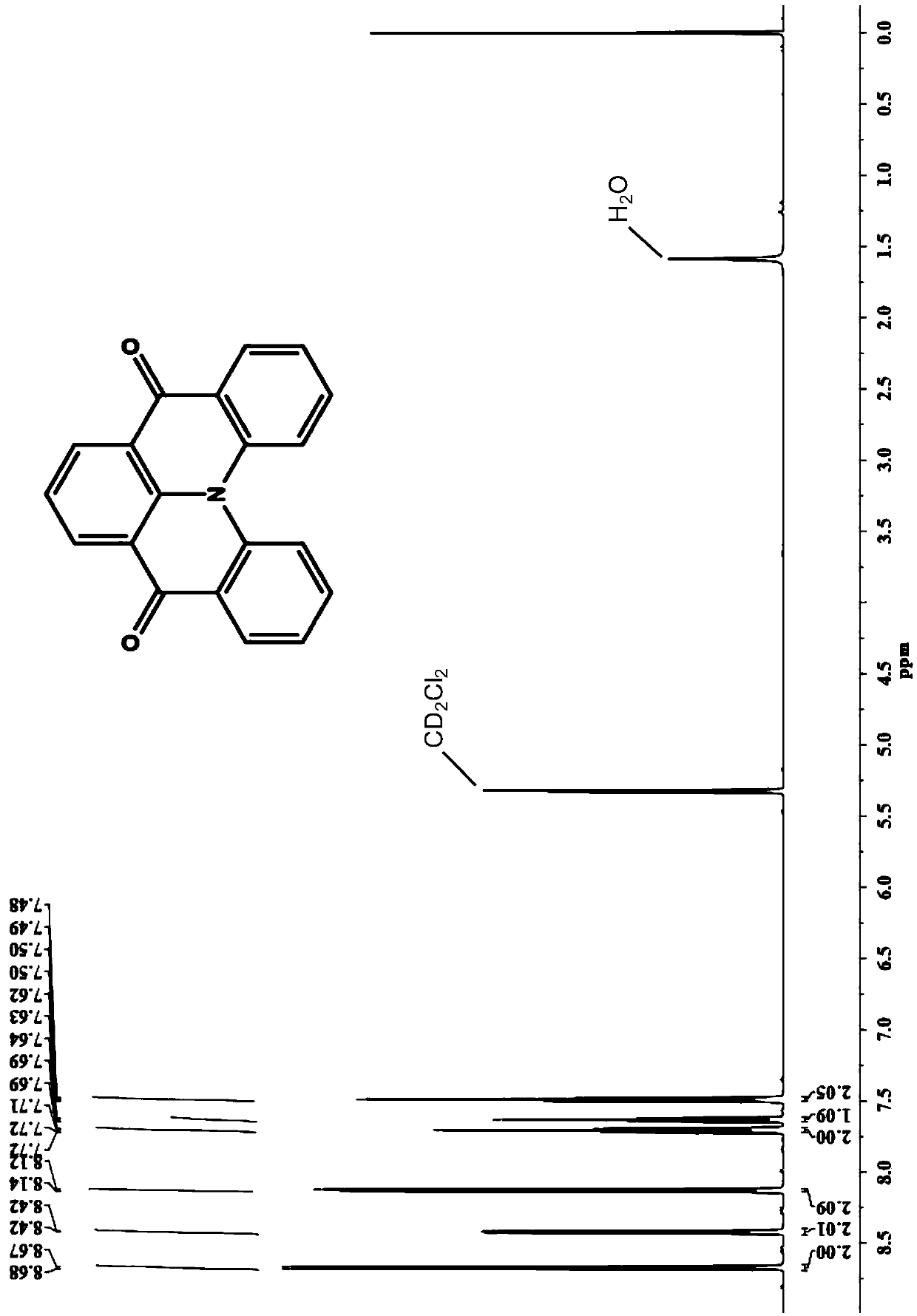
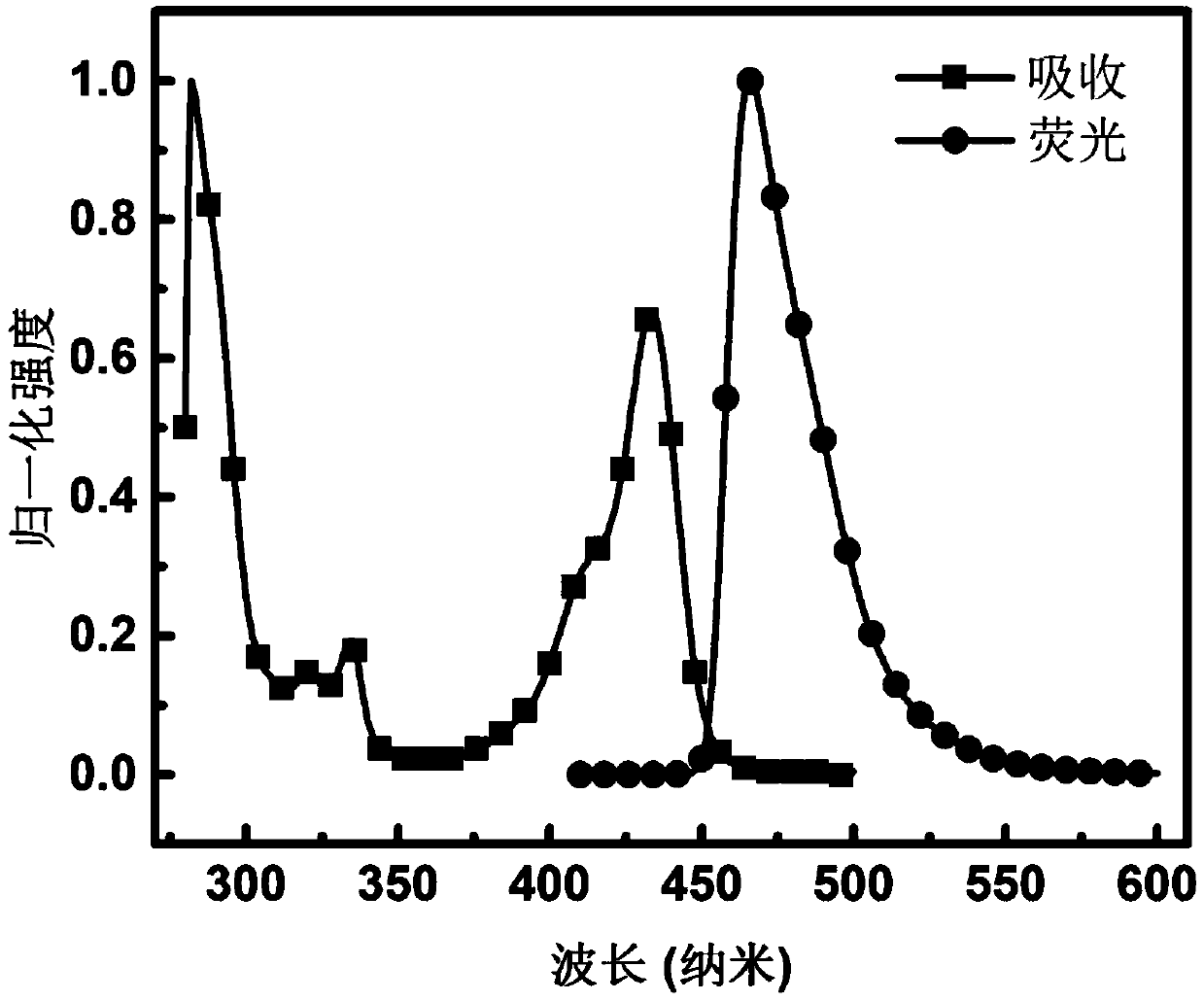
Organic light emitting device, carbonyl bridged triarylamine derivative and application of carbonyl bridged triarylamine derivative
A carbonyl bridged, triarylamine technology, applied in the field of organic electroluminescence devices, achieves the effects of low preparation cost, flexible derivatization methods, and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The synthesis of embodiment 1 compound C-001
[0063]
[0064] The preparation method of formula i1 compound is: add 4.8g (15mmol) methyl 2-iodoisophthalate, 2.71g (16mmol) diphenylamine, 2.49g (18mmol) salt of wormwood, 0.19g ( 3mmol) of activated copper powder and 100mL o-dichlorobenzene, degas the reaction system, then protect it with argon, stir and heat to 180°C and continue the reaction for 48h. After the reaction is complete, cool the system to room temperature, filter under reduced pressure and wash the filter residue with dichloromethane. After the filtrate is spin-dried, use dichloromethane:petroleum ether=1:1 (volume ratio) eluent on a silica gel column Separation and purification were carried out to obtain 4.53 g of light yellow-green oily liquid with a yield of 83.5%. MS(EI):m / z 361.12[M + ].
[0065] The preparation method of the compound of formula i2 is: in a 250mL round-bottomed flask, 3.61g (10mmol) i1, 2.0g (50mmol) sodium hydroxide are dissolve...
Embodiment 2
[0067] The synthesis of embodiment 2 compound C-026a
[0068]
[0069] The synthesis of formula i3 compound: add 4.31g (15mmol) 2-bromo-5-methyl isophthalic acid methyl esters, 2.71g (16mmol) diphenylamine, 2.49g (18mmol) potassium carbonate successively in 250mL round bottom flask, 0.19g (3mmol) activated copper powder and 100mL o-dichlorobenzene, degas the reaction system, then protect it with argon, stir and heat to 180°C and continue the reaction for 48h. After the reaction is complete, cool the system to room temperature, filter under reduced pressure and wash the filter residue with dichloromethane, spin the filtrate to dryness, and use dichloromethane:petroleum ether=3:2 (volume ratio) eluent Separation and purification were carried out to obtain 4.9 g of light yellow-green oily liquid with a yield of 87.1%. MS(EI):m / z 375.11[M + ].
[0070] The synthesis of formula i4 compound: in the round-bottomed flask of 100mL, under the condition of ice bath and light-shield...
Embodiment 3
[0074] The synthesis of embodiment 3 compound C-037a
[0075]
[0076] The preparation method of formula i7 compound is: add 5.98g (15mmol) 5-bromo-2-iodomethyl isophthalate, 2.71g (16mmol) diphenylamine, 2.49g (18mmol) potassium carbonate successively in 250mL round bottom flask , 0.19g (3mmol) of activated copper powder and 100mL of o-dichlorobenzene, degas the reaction system, then protect it with argon, stir and heat to 180°C and continue the reaction for 48h. After the reaction is complete, cool the system to room temperature, filter under reduced pressure and wash the filter residue with dichloromethane. After the filtrate is spin-dried, use dichloromethane:petroleum ether=1:1 (volume ratio) eluent on a silica gel column Separation and purification were carried out to obtain 4.73 g of light yellow solid with a yield of 71.6%. MS(EI):m / z 439.58[M+ ].
[0077] The preparation method of the compound of formula i8 is: in a 250mL round-bottomed flask, 4.39g (10mmol) i7, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com