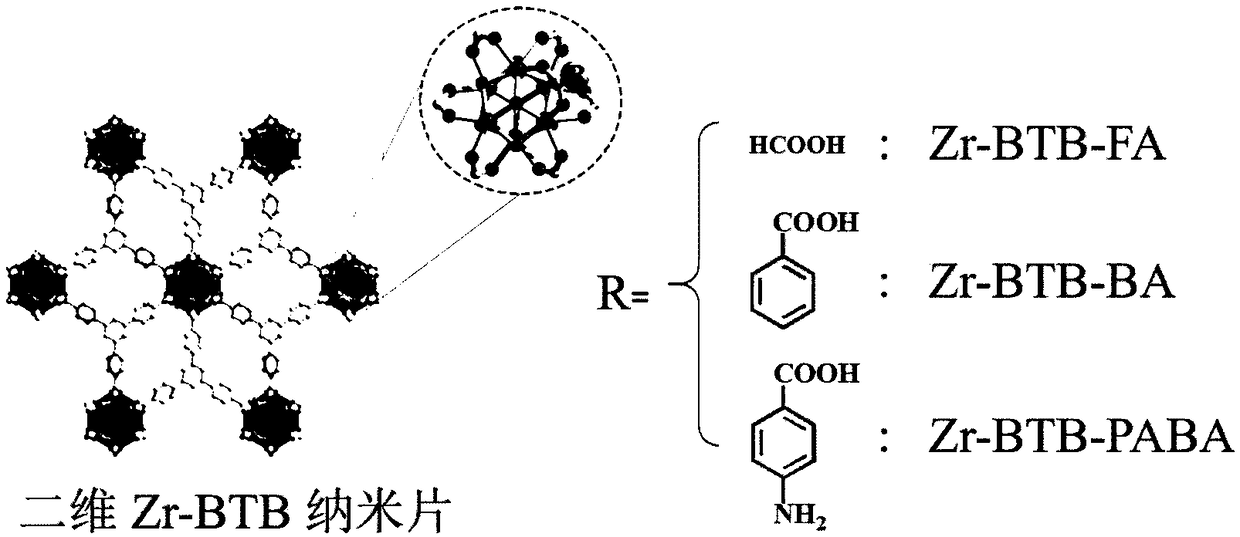
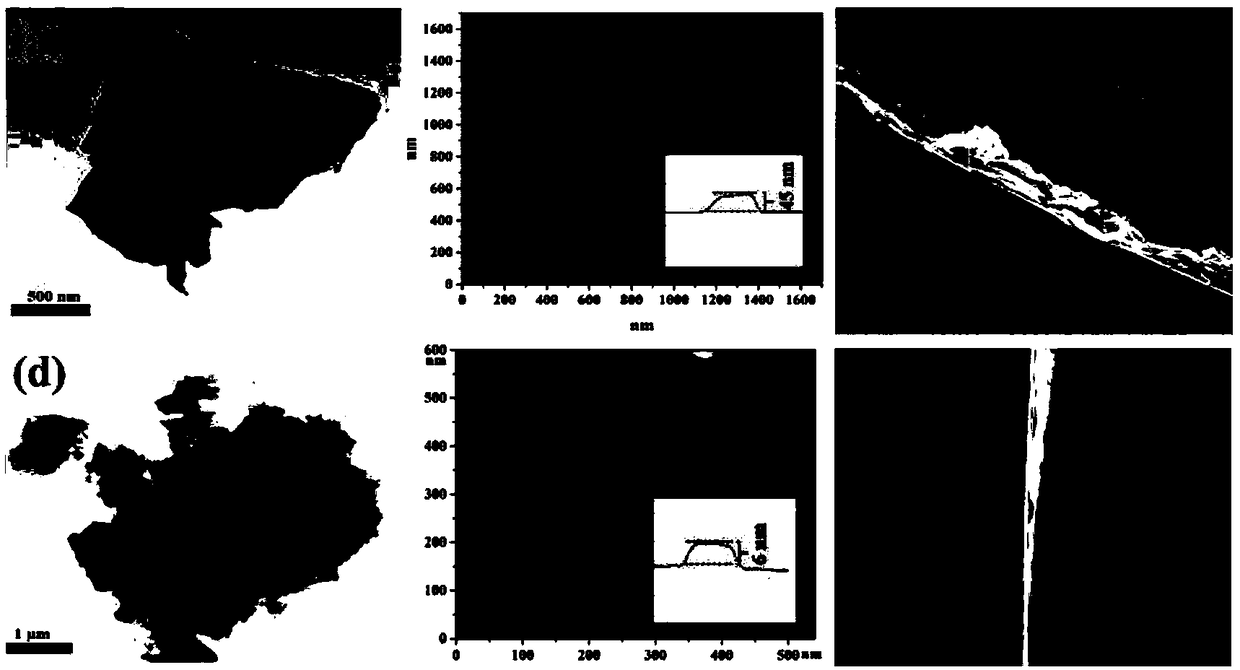
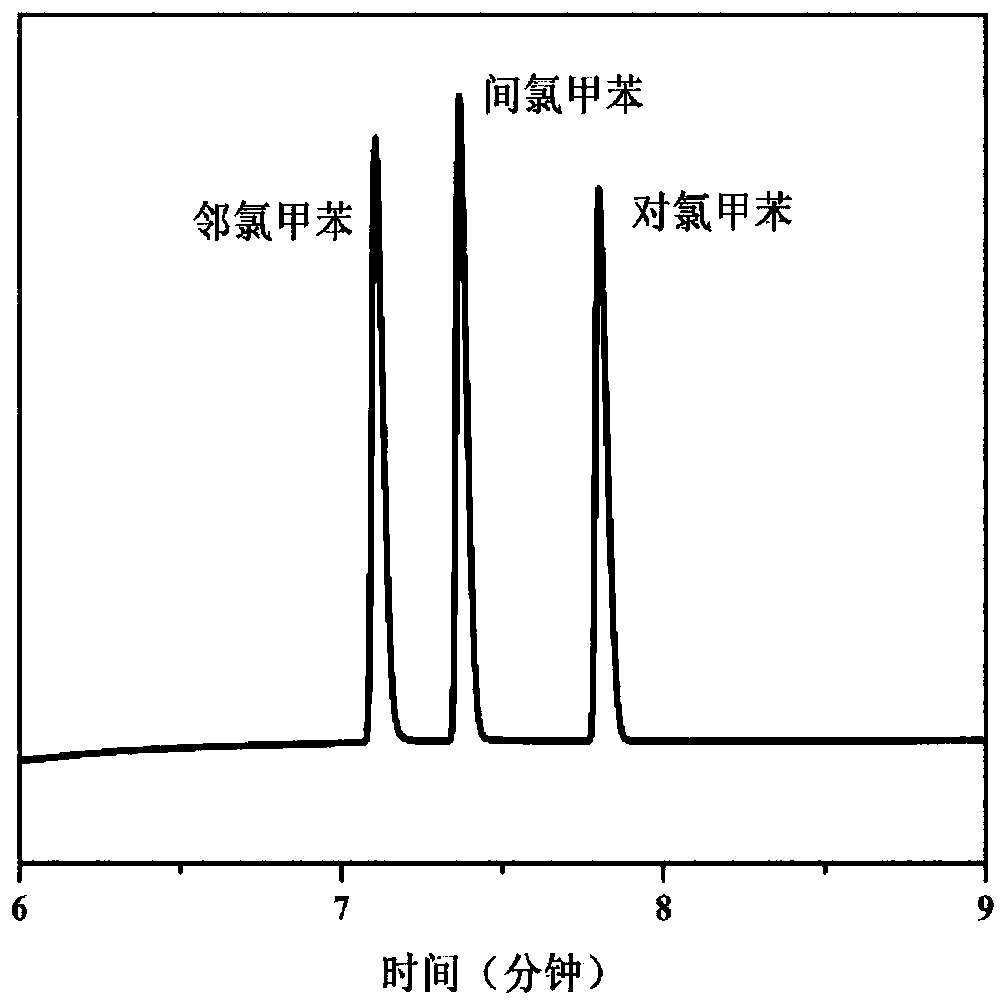
Two-dimensional metal organic framework nanosheet-based capillary gas chromatography column and preparation method and application thereof
A technology of metal-organic framework and gas chromatography column, which is applied in the field of chromatographic separation, can solve problems such as the limitation of the range of separable target compounds, and achieve the effects of significant production and practical significance, improved separation, and large specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Add 10.12mg of zirconium tetrachloride, 12.50mg of 1,3,5-tris(4-carboxyphenyl)benzene, 5mL of N,N-dimethylformamide solvent and sonicate for 10 minutes in a 22mL glass upright vial, and then Add 1.11 g of formic acid and 60 μL of water, seal the bottle and cover it, and heat the glass vial in an oven at 120° C. for 48 hours. After cooling down to room temperature, centrifuge at 12,000 rpm to obtain white nanosheets, soak in N, N-dimethylformamide and ethanol solutions in turn and then wash to remove unreacted reagents in the pores of the nanosheets. Finally, the product was vacuum-dried and activated at 80°C for 12 hours, and the prepared nanosheets were labeled as multilayer Zr-BTB-FA.
[0042] Take 10 mg of multilayer Zr-BTB-FA nanosheet powder and disperse it in 3 mL of ethanol solution, ultrasonicate for 30 minutes to obtain a uniform suspension, take 1 mL of the above suspension in a syringe, slowly pour it into the pretreated capillary column, and then Driven by ...
Embodiment 2
[0052] Add 10mg zirconium tetrachloride, 10mg 1,3,5-tris(4-carboxyphenyl)benzene, 3mL N,N-dimethylformamide solvent and sonicate for 10 minutes, then add 600mg Benzoic acid and 250 μL of water, seal the bottle and cover it, and place the glass vial in an oven at 120°C for 48 hours. After cooling down to room temperature, centrifuge at 12,000 rpm to obtain white nanosheets, soak in N, N-dimethylformamide and ethanol solutions in turn and then wash to remove unreacted reagents in the pores of the nanosheets. Finally, the product was vacuum-dried and activated at 80°C for 12 hours, and the prepared nanosheets were labeled as multilayer Zr-BTB-BA.
[0053] The process of the capillary acid-base pretreatment and amino functional pretreatment is the same as above.
[0054] Take 10 mg of multilayer Zr-BTB-BA nanosheet powder and disperse it in 3 mL of ethanol solution, and ultrasonicate for 30 minutes to obtain a uniform suspension, take 1 mL of the above suspension in a syringe, sl...
Embodiment 3
[0057] Add 10.12mg of zirconium tetrachloride, 12.50mg of 1,3,5-tris(4-carboxyphenyl)benzene, 5mL of N,N-dimethylformamide solvent and sonicate for 10 minutes in a 22mL glass upright vial, and then Add 1.11 g of formic acid and 60 μL of water, seal the bottle and cover it, and heat the glass vial in an oven at 120° C. for 48 hours. After cooling down to room temperature, centrifuge at 12,000 rpm to obtain white nanosheets, soak in N,N-dimethylformamide solution for several times and then wash, take 30mL of N,N-dimethylformamide solution containing about 100mg of nanosheets Add 1000 mg p-aminobenzoic acid ligand to a single-necked round-bottom glass flask for post-synthesis modification, and heat the mixture at 120°C for 72 hours under oil bath conditions to fully bond the post-modification ligand p-aminobenzoic acid on the zirconium metal clusters . The solution was cooled to room temperature, centrifuged at 12,000 rpm to obtain white nanosheet solids, soaked in N, N-dimethyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com