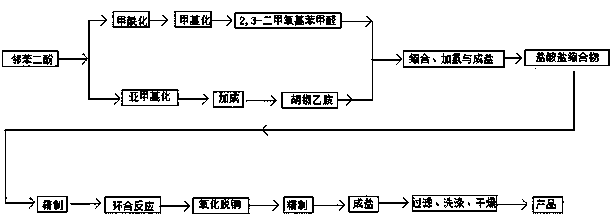
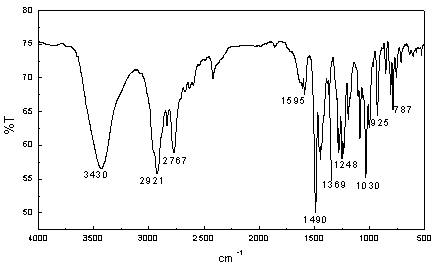
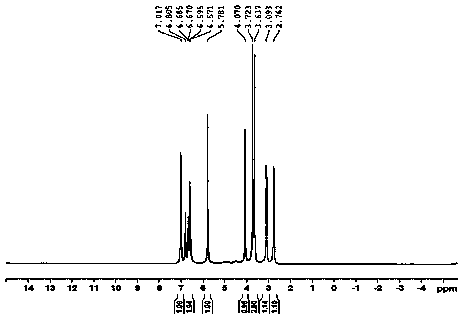
Method for fully synthesizing berberine
A berberine, total synthesis technology, applied in the direction of drug combination, metabolic diseases, cardiovascular system diseases, etc., can solve the problems of ineffective control of impurity components and content, unclear related substances, and many reaction steps, so as to achieve the treatment of Alzheimer's disease. Zheimer's disease, the effect of reducing patient suffering and saving time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0037] (1) Weigh catechol and 2-chloromethane according to the molar ratio, put them into a 500ml three-necked flask with dimethyl sulfoxide as the solvent, add an acid-binding agent (alkali), react at 130°C for 6 hours, reduce Part of the 2-chloromethane was recovered by pressure distillation, and the product at 130-135°C was collected to obtain the target product piperonine.
[0038] (2) Using ethyl acetate as a solvent, weigh piperonylcycline and 2-chloroethylamine according to the molar ratio, put them into a 500ml three-neck flask, add catalyst, add 2-chloroethylamine dropwise, stir for 8 hours, and after cooling, reduce Ethyl acetate was obtained by pressing, crystallized at -10°C, vacuum filtered, and recrystallized from ethanol to obtain piperonylethylamine.
[0039] (3) According to the mass ratio of 1:4, weigh catechol and sodium hydroxide, put them into a 500ml three-necked flask, add catalyst (alcohol), use methanol as solvent, heat up and stir until the solid diss...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com