Double-metal material supported phosphotungstic acid catalyst as well as preparation method and application thereof to cellulose hydrolysis
A phosphotungstic acid and catalyst technology, applied in the field of materials, can solve the problems of structural collapse, MIL-101 structural stability and water stability obstacles, and achieve the effects of preventing loss, maintaining acid catalytic activity, and improving water stability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
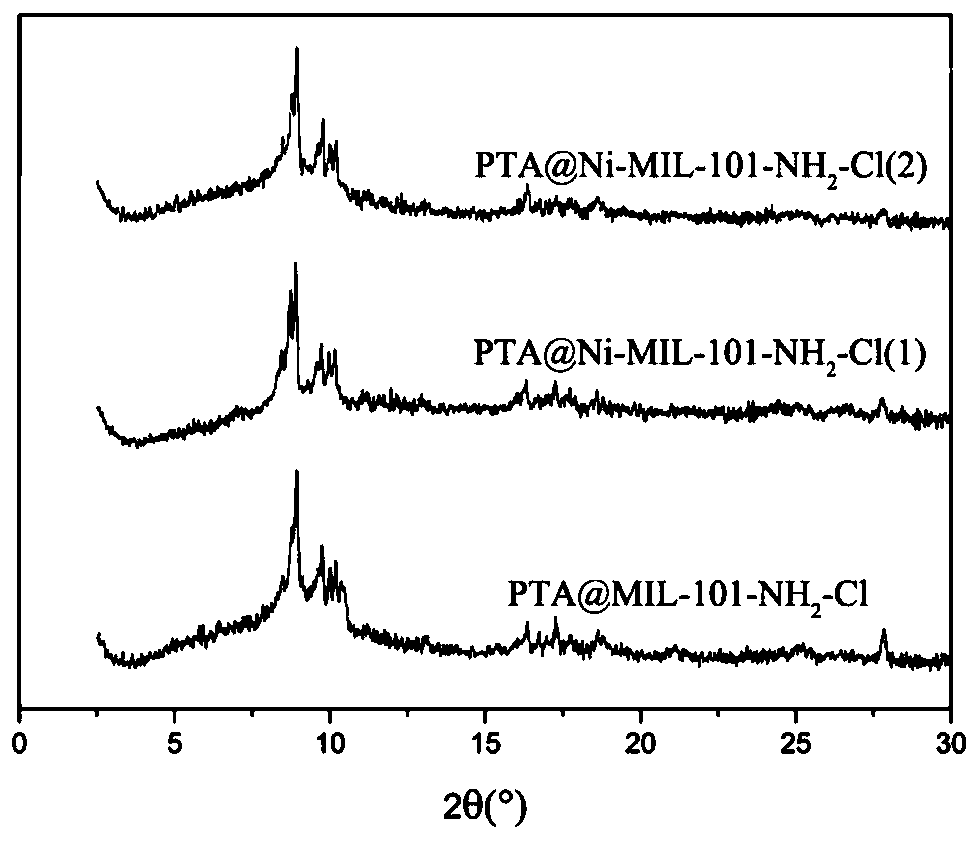
[0030] Composite bimetallic Ni / MIL-101 (Cr) synchronous immobilized phosphotungstic acid catalyst is prepared by the following method: Weigh the central metal ion Cr respectively 3+ and metal ions Ni 2+ Metal salts of phosphotungstic acid (PTA), terephthalic acid, 2,5-dichloroterephthalic acid (TA-Cl) and 2-aminoterephthalic acid (TA-NH 2 ), Cr 3+ The amount is 2mmol, Ni 2+The amount of Ni / (Cr+Ni)=0.2 is 0.5 mmol. The amounts of phosphotungstic acid, terephthalic acid, 2,5-dichloroterephthalic acid and 2-aminoterephthalic acid were 2mmol, 1mmol, 0.5mmol, 0.5mmol respectively, and 0.35mmol of citric acid was added, and Mix in 70mL of pure water and sonicate, transfer the sonicated solution to a polytetrafluoroethylene reactor and heat at a constant temperature of 220°C for 8h, wait to cool to room temperature, centrifuge the resulting solution, and use N,N-dimethyl Wash the solid twice with formamide, each washing time is 1 h, and continue to wash the solid several times wi...
Embodiment 2
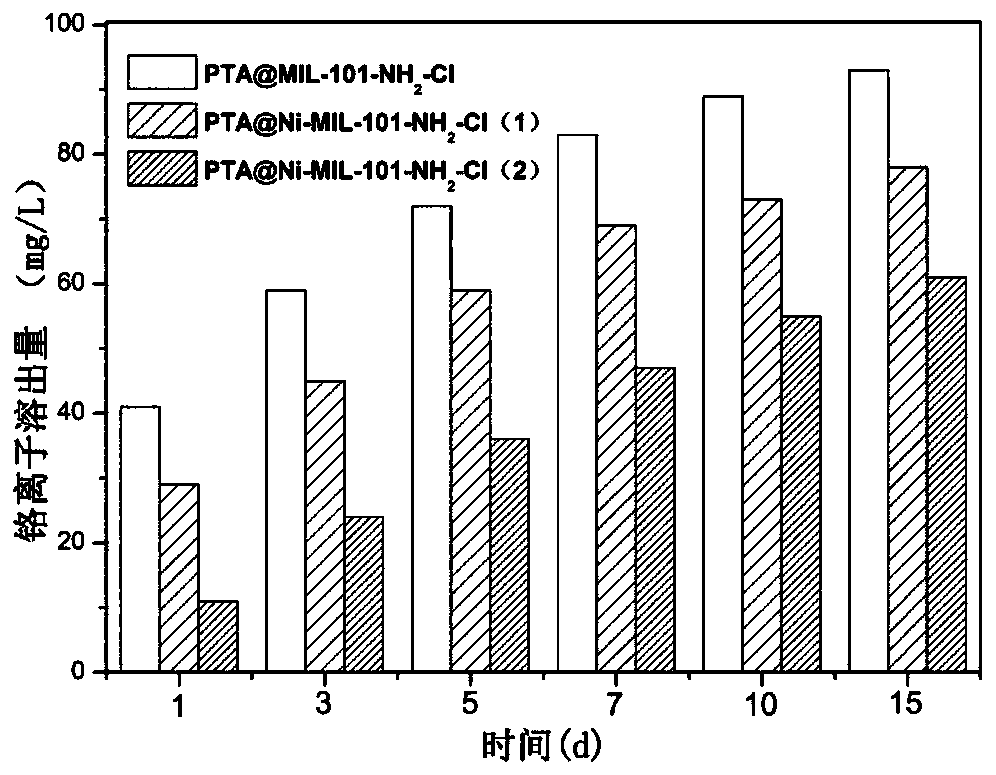
[0033] Synthesize material PTA@MIL-101-NH according to the step in embodiment 1 2 -Cl, PTA@Ni-MIL-101-NH 2 -Cl(1), PTA@Ni-MIL-101-NH 2 -Cl(2), respectively weigh 1g of the above three materials into a 100mL saline bottle, add 50mL of distilled water and mix well, then let it stand at room temperature. Take the supernatant at the 1st, 3rd, 5th, 7th, 10th, and 15th day respectively, filter through a 0.22 μm filter membrane and measure its chromium ion content by ion chromatography, which is the chromium ion leaching concentration of the material in distilled water, which is used to reflect Material stability in water. PTA@MIL-101-NH 2 -Cl, PTA@Ni-MIL-101-NH 2 -Cl(1), PTA@Ni-MIL-101-NH 2 -Chromium ion leaching concentration of Cl(2) in distilled water changes with leaching time.
[0034] Such as figure 2 As shown, it can be seen that the chromium ion leaching concentration of the three materials increases with the prolongation of the leaching time, and PTA@MIL-101-NH 2 T...
Embodiment 3
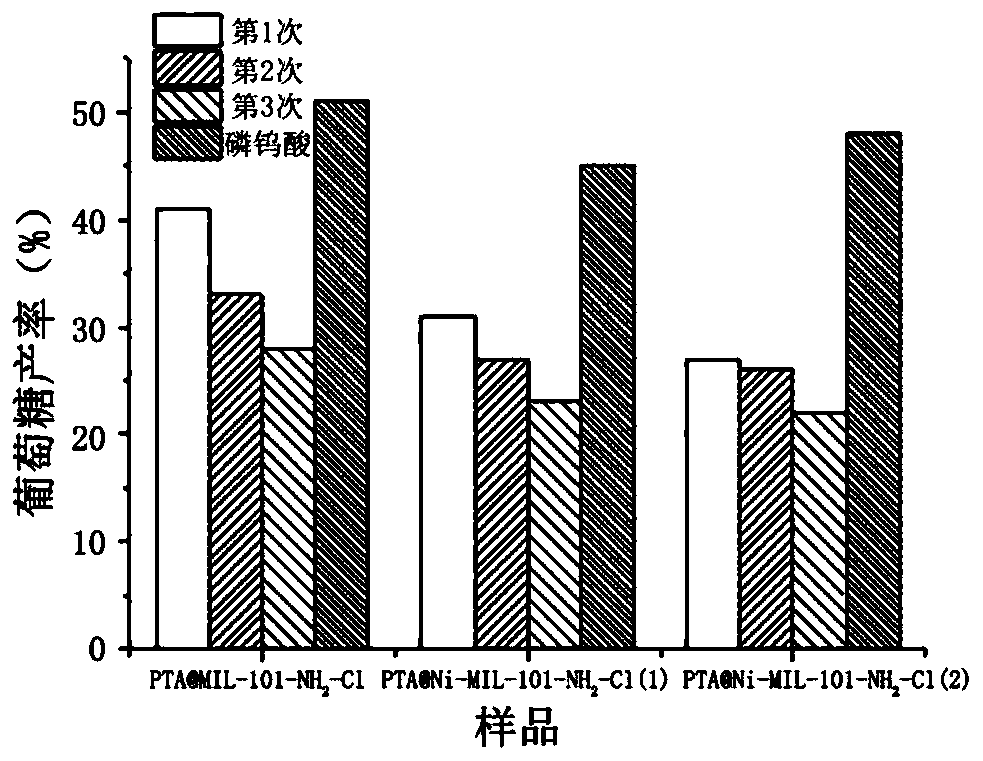
[0036] Synthesize material PTA@MIL-101-NH according to the step in embodiment 1 2 -Cl, PTA@Ni-MIL-101-NH 2 -Cl(1), PTA@Ni-MIL-101-NH 2 -Cl(2), applying the phosphotungstic acid catalyst to cellulose hydrolysis, comprising the following steps: adding 0.12g of PTA@Ni-MIL-101-NH to 10mL distilled water 2 -Cl (add the same 0.12g of PTA@MIL-101-NH in the control experiment 2 The same amount of pure phosphotungstic acid catalyst in -Cl), and 0.04 g of microcrystalline cellulose were added at the same time, heated at a constant temperature at 180 ° C and continuously stirred for 11 h, and centrifuged after the reaction. The solid residue precipitated at the bottom of the centrifuge tube after centrifugation is the large-pore metal-organic framework material of immobilized PTA used to catalyze the hydrolysis of microcrystalline cellulose after the reaction. It can be reused after washing with distilled water and drying at 80°C for 16 hours .
[0037] Under the same test conditions...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com