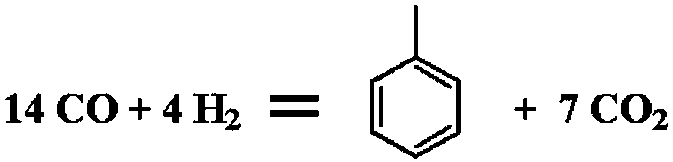
Method for directly preparing p-xylene from synthesis gas and methylbenzene
A technology for para-xylene and synthesis gas, which is used in chemical instruments and methods, condensation between hydrocarbons and non-hydrocarbons to produce hydrocarbons, catalyst activation/preparation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
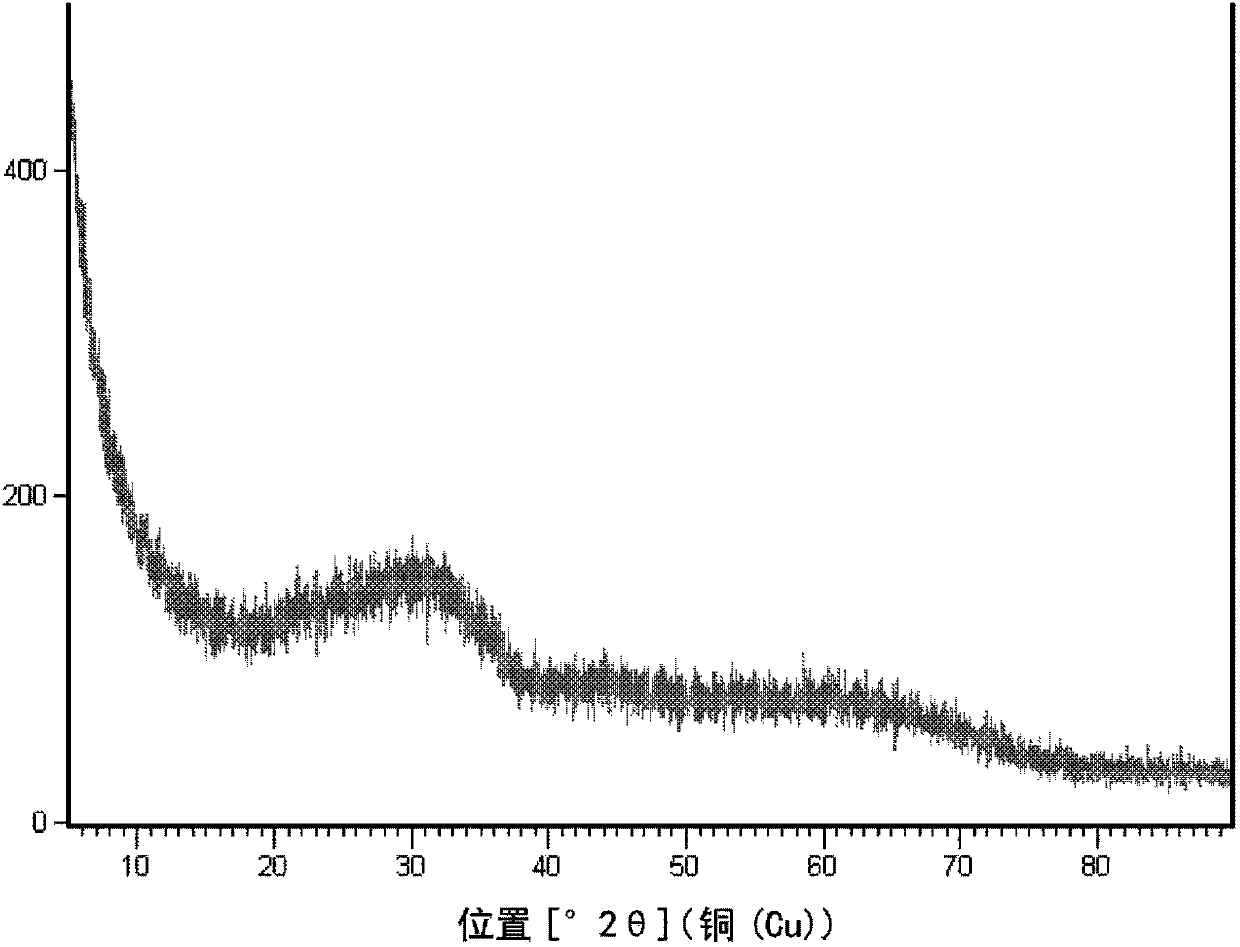
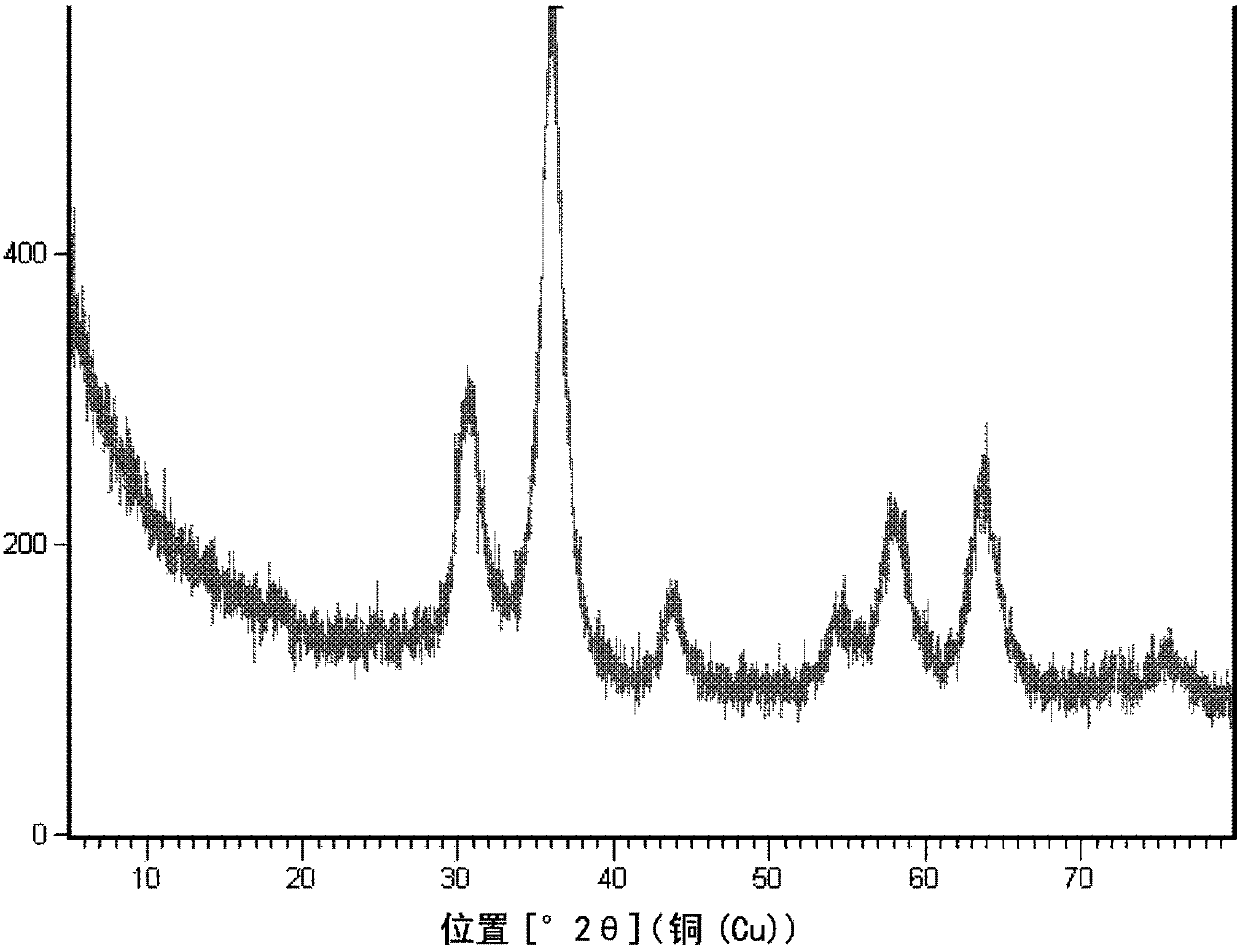
[0091] Prepared with 0.05mol / L Zn 2+ With 1.0mol / L Al 3+ 1 L of mixed nitrate aqueous solution, and slowly add 0.5 mol / L ammonia solution to it, while controlling the coprecipitation reaction temperature to be 70°C and controlling the pH value to be 7.2, so as to co-precipitate metal ions. After the reaction, the reaction mixture was aged at 70° C. for 4 h. The precipitate was filtered, washed with deionized water, dried, and calcined at 500°C for 4 hours to obtain a highly dispersed zinc oxide material with alumina as an inert carrier, coded as A. A contains 8.3% by weight of zinc, and the XRD pattern is as follows figure 1 shown.
Embodiment 2
[0093] Prepared with 0.02mol / L Zn 2+ , 0.02mol / L Cr 3+ With 1.0mol / L Al 3+ Add 1 L of mixed nitrate aqueous solution, and slowly add 1.0 mol / L ammonium carbonate solution to it, while controlling the co-precipitation reaction temperature to 70°C and controlling the pH value to 7.5, so as to co-precipitate metal ions. After the reaction, the reaction mixture was aged at 70° C. for 4 h. The precipitate was filtered, washed with deionized water, dried, and calcined at 500°C for 4 hours to obtain a highly dispersed zinc-chromium oxide material with alumina as an inert carrier, coded as B. B contains 3.1% by weight of zinc and 2.5% by weight of chromium.
Embodiment 3
[0095] Prepared with 0.01mol / L Zn 2+ , 0.01mol / L Zr 4+ With 1.0mol / L Al 3+ Add 1 L of mixed nitrate aqueous solution, and slowly add 1.2 mol / L sodium carbonate solution into it, while controlling the co-precipitation reaction temperature to 70°C and controlling the pH value to 7.3, so as to co-precipitate metal ions. After the reaction, the reaction mixture was aged at 70° C. for 4 h. The precipitate was filtered, washed with deionized water, dried, and calcined at 500°C for 4 hours to obtain a highly dispersed zinc-zirconium oxide material with alumina as an inert carrier, coded as C. C contains 1.5% by mass of zinc and 2.1% by mass of zirconium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com