Continuous synthesis method of diazoacetate
A technology of diazoacetate and synthesis method, applied in the direction of organic chemistry, etc., can solve the problems of high ammonia nitrogen and high salt wastewater discharge, low biochemical efficiency of wastewater, difficult biochemical treatment, etc., and achieve high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
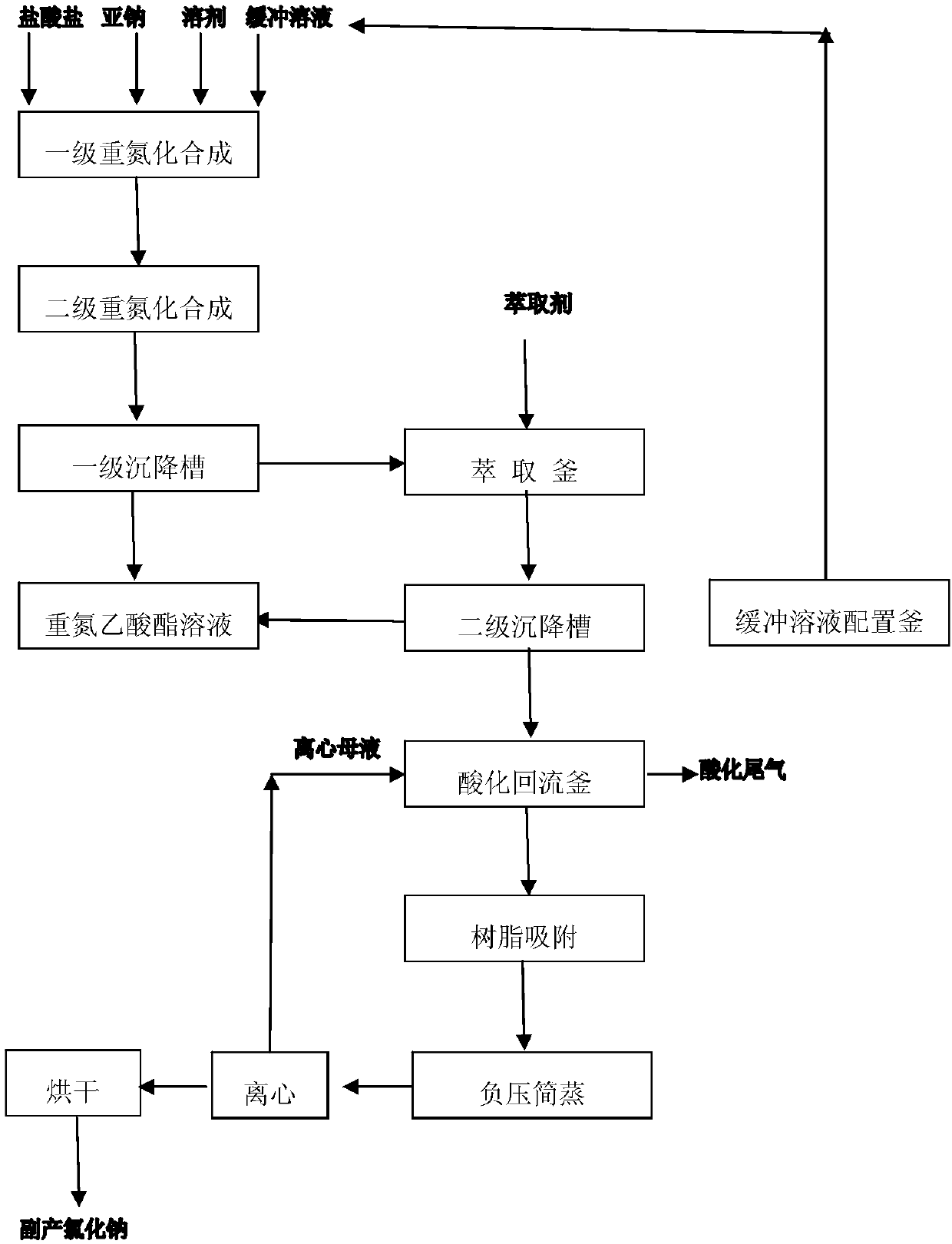
[0027] A clean production method for continuous synthesis of pyrethroid intermediate diazoacetate:
[0028] (1) 75kg hydrochloride aqueous solution (glycine methyl ester hydrochloride and 0.1M acetic acid / sodium acetate buffer solution is prepared, the pH value is adjusted to 4.3, wherein the mass fraction of glycine methyl ester hydrochloride is 35%), 45kg sodium nitrite aqueous solution (sodium nitrite mass fraction is 35%), 70kg solvent chloroform is put into the first-level diazotization synthesis kettle together, wherein, the glycine methyl ester hydrochloride in the hydrochloride aqueous solution and the sodium nitrite aqueous solution The molar ratio of sodium nitrite in the solution is 1:1.09; the primary diazotization synthesis is carried out at 12°C, and the reaction is 4h; the primary diazotization synthesis product overflows into the secondary diazotization synthesis kettle, The secondary diazotization synthesis is carried out under the lower pressure, and the reac...
Embodiment 2
[0032](1) 8kg aqueous hydrochloride solution (formed from ethyl glycine hydrochloride and 0.1M acetic acid / sodium acetate buffer solution, the pH value is adjusted to 4.2, wherein the mass fraction of ethyl glycine hydrochloride is 40%), 45kg of sodium nitrite aqueous solution (sodium nitrite mass fraction is 45%), 45kg of solvent 1,2-ethylene dichloride are put into the primary diazotization synthesis kettle together, wherein, glycine ethyl ester hydrochloride in hydrochloride aqueous solution The molar ratio of salt to nitrous acid in the sodium nitrite aqueous solution is 1:1.28; the primary diazotization synthesis is carried out at 5°C, and the reaction is 2h; the primary diazotization synthesis product overflows into the secondary diazotization synthesis kettle , carry out two-stage diazotization synthesis at 5°C, and the reaction time is 2 hours; the secondary diazotization synthesis product overflows into the first-stage settling tank for static stratification, the oil l...
Embodiment 3
[0035] (1) 80kg hydrochloride aqueous solution (prepared from formic acid / sodium formate buffer solution of glycine ethyl ester hydrochloride and 0.1M, the pH value is adjusted to 4.3, wherein the mass fraction of glycine ethyl ester hydrochloride is 40%), 40kg Sodium nitrite aqueous solution (sodium nitrite mass fraction is 50%), 60kg solvent dichloromethane is put into one-stage diazotization synthesis kettle together, wherein, glycine ethyl ester hydrochloride in the hydrochloride aqueous solution and sodium nitrite aqueous solution The molar ratio of nitrous acid is 1:1.26; the primary diazotization synthesis is carried out at 3°C, and the reaction is 1h; Two-stage diazotization synthesis, the reaction time is 3h; the secondary diazotization synthesis product overflows into the first-stage settling tank for static stratification, the oil layer goes to the low-level tank for diazotization, and the water layer overflows to the extraction kettle; Add 60kg of extractant dichlo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com