A kind of continuous synthesis method of diazoacetate
A technology of diazoacetate and synthesis method, applied in the direction of organic chemistry and the like, can solve the problems of high ammonia nitrogen and high salt-containing wastewater discharge, low biochemical efficiency of wastewater, difficult biochemical treatment and other problems, and achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
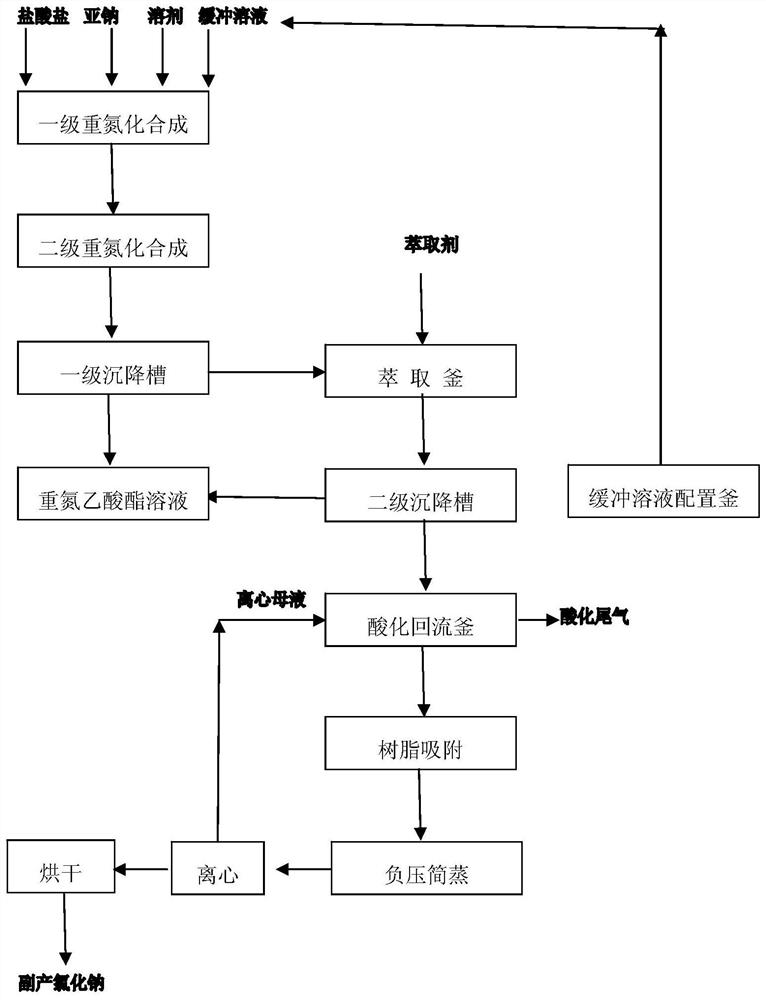
[0027] A pyrethroid intermediate diazoacetate continuous synthesis clean production methods:
[0028] (1) A 75kg hydrochloride aqueous solution (sodium glycine methyl ester hydrochloride and 0.1M acetic acid / acetate buffer solution preparation, pH value was adjusted to 4.3, wherein the salt of glycine methyl ester hydrochloride mass fraction of 35%), aqueous sodium 45kg (mass fraction of sodium nitrite 35%), placed in a chloroform solvent with 70kg diazotization synthesis reactor, in which the glycine methyl ester hydrochloride aqueous hydrochloric acid and aqueous sodium salt sodium nitrite in a molar ratio of 1: 1.09; synthesis of a diazotized at 12 ℃, 4H reaction; an overflow to a secondary synthesis product diazotized diazonium synthesis reactor, the 8 ℃ carried out under two diazotization synthesis, the reaction time is 4H; two diazotized synthesis product overflows into a settling tank for standing layered reservoir to the lower tank diazonium night, the aqueous layer was ...
Embodiment 2
[0032](1) The 8kg hydrochloride aqueous solution (sodium glycine ethyl ester hydrochloride and 0.1M acetic acid / acetate buffer solution preparation, pH value was adjusted to 4.2 with glycine ethyl ester hydrochloride salt mass fraction of 40%), aqueous sodium 45kg (mass fraction of sodium nitrite 45%), 1,2-dichloroethane solvent together into a diazonium 45kg synthesis reactor, in which the aqueous hydrochloric acid salt of glycine ethyl ester hydrochloride the molar ratio of salt to an aqueous solution of sodium nitrite is 1: 1.28; synthesis of a diazotized at 5 ℃, 2H reaction; an overflow to a secondary diazotized synthesis product in the synthesis reactor diazotization carried out at 5 ℃ two diazotization synthesis, the reaction time is 2H; two diazotized synthesis product overflows into a settling tank for standing layered reservoir to the lower tank diazonium night, the aqueous layer underflow flow to the extraction vessel; 1,2-dichloroethane was added 60kg extractant to th...
Embodiment 3
[0035] (1) A 80kg hydrochloride solution (0.1M glycine ethyl ester hydrochloride and formic acid / sodium formate buffer solution preparation, pH value was adjusted to 4.3, wherein the salt of glycine ethyl ester hydrochloride mass fraction of 40%), 40kg an aqueous solution of sodium (mass fraction of sodium nitrite 50%), into the synthesis reactor together with a diazotized 60kg solvent such as dichloromethane, in which aqueous hydrochloric acid ethyl ester hydrochloride salt with aqueous sodium glycine alkylene molar ratio of nitrous acid is 1: 1.26; diazotized at a 3 ℃ synthesis, the reaction IH; an overflow to a secondary synthesis product diazotized diazonium synthesis pot, and at 3 ℃ two diazotization synthesis, the reaction time is 3H; two diazotized synthesis product overflows into a settling tank for standing layered reservoir to the lower tank diazonium night, the aqueous layer was extracted kettle to overflow; to 60kg kettle was added and extracted with dichloromethane ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com