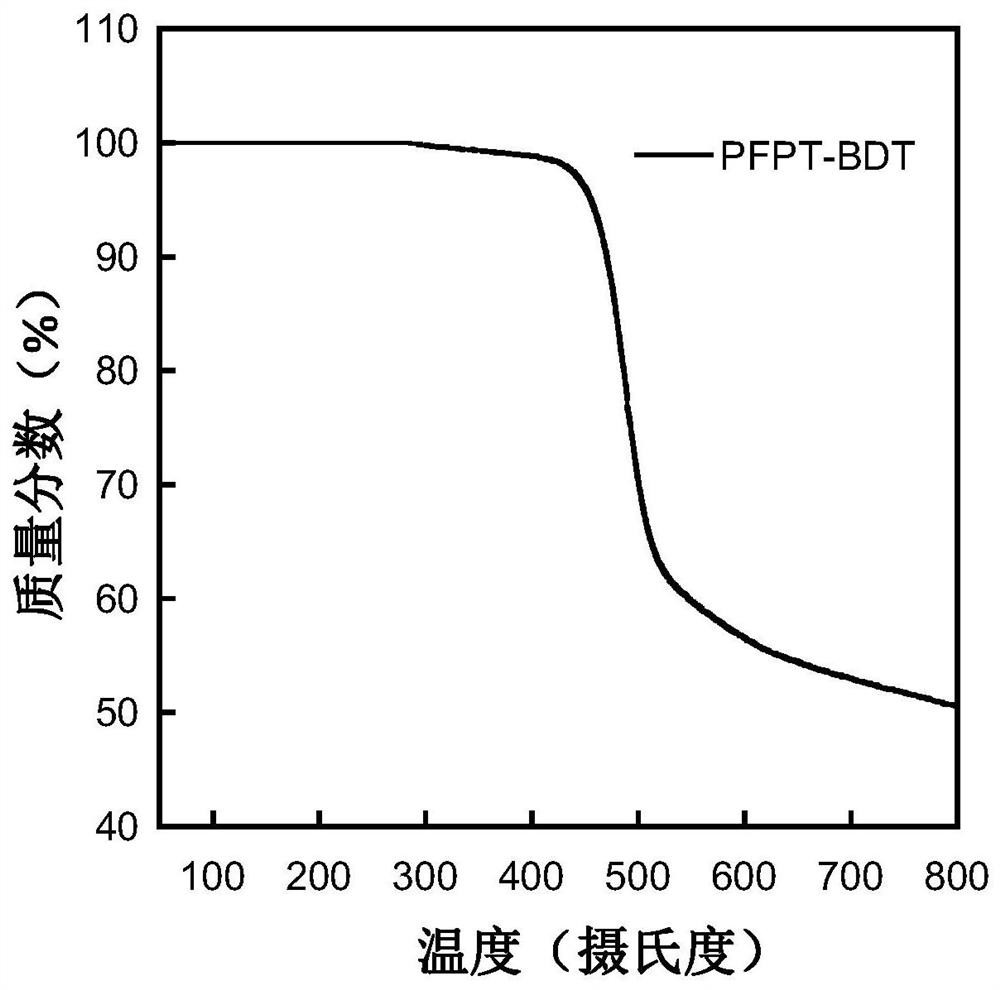
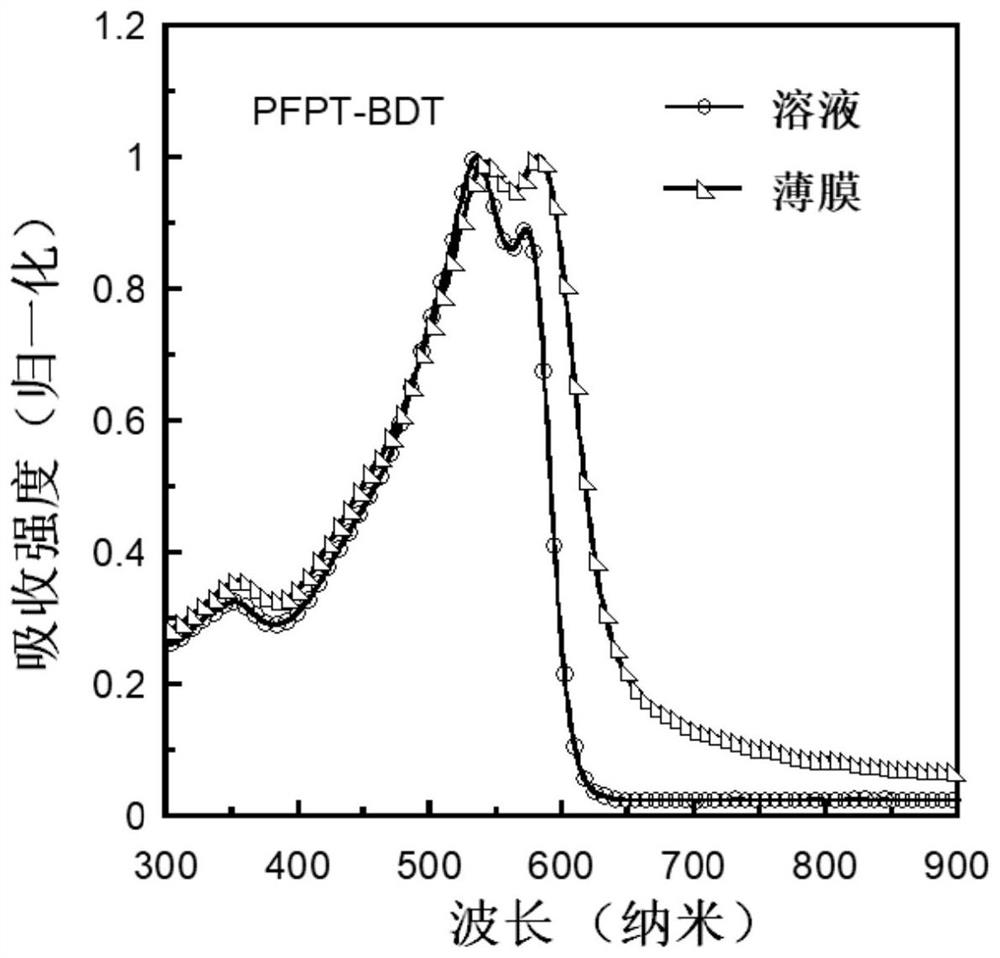
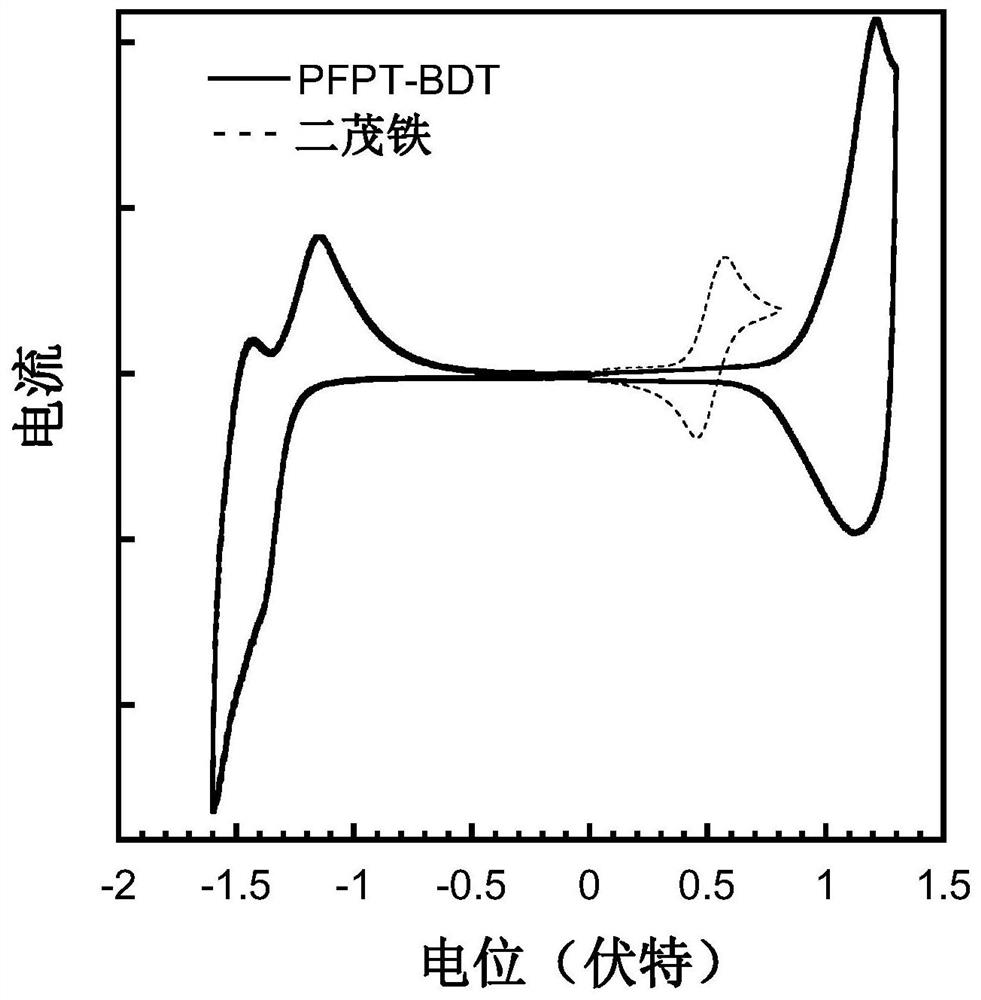
A kind of conjugated polymer containing difluoronaphthothiophenedione electron-withdrawing unit and its synthesis method and application
A technology of conjugated polymer and synthesis method, applied in the field of polymers, can solve problems such as less material, and achieve the effects of high mobility, good solution processing performance, and excellent optoelectronic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] 1,3-bis(2-ethylhexyl)-6,7-difluoro-5,8-bis(thiophen-2-yl)naphtho[2,3-c]thiophene-4,9-dione preparation:
[0035] (1) Under nitrogen protection, add 4,5-difluorophthalic acid 1 (4.04g, 20mmol) and a drop of N,N-dimethylformamide (DMF) into a 100mL two-necked round-bottomed flask, Oxalyl chloride (10 mL), and then add 20 mL of anhydrous dichloromethane as a solvent, and stir at room temperature for 12 hours. 2,5-bis(2-ethylhexyl)thiophene 2 (6.17 g, 20 mmol), AlCl 3 (10.67g, 80mmol), it was moved to 0°C under an ice-water bath and stirred for 30 minutes, then placed at room temperature and stirred for 3 hours. Post-processing: the reaction mixture was extracted with dilute hydrochloric acid aqueous solution and chloroform to obtain an organic phase, which was put into a one-port bottle and mixed with silica gel powder for rotary evaporation, and then purified with a silica gel column (petroleum ether: dichloromethane was selected as eluent). 4:1 (v / v)), the product 3 w...
Embodiment 2
[0042] 1,3-bis(2-ethylhexyl)-6,7-difluoro-5,8-bis(5-bromothien-2-yl)naphtho[2,3-c]thiophene-4,9 - Preparation of diketones (M1):
[0043] In a 100 mL single-necked bottle, compound 6 (0.69 g, 1 mmol) was dissolved in a mixed solvent of chloroform (30 mL) and acetic acid (30 mL), and the volume ratio of chloroform and acetic acid was 1:1. Then the one-necked flask was placed in an ice bath, and when the temperature of the reaction solution dropped to about 0°C, N-bromosuccinimide NBS (0.38 g, 2 mmol) was added batch by batch, and the reaction was stirred at room temperature for 8 hours. Post-processing: Pour the reaction solution into water, extract with dichloromethane, wash the organic layer with saturated aqueous sodium chloride solution, and dry over anhydrous magnesium sulfate. After distillation under reduced pressure, the crude product was purified by silica gel column (petroleum ether was selected as the eluent) to obtain the yellow solid product M1 (1,3-bis(2-ethylhex...
Embodiment 3
[0046] Preparation of PFPT-BDT
[0047] In the water-oxygen automatic control glove box, compound M1 (1,3-bis(2-ethylhexyl)-6,7-difluoro-5,8-bis(5-bromothiophen-2-yl)naphthol [2,3-c]thiophene-4,9-dione) (79.7mg, 0.10mmol), compound M2 (4,8-bis(5-(2-ethylhexyl)thiophen-2-yl)benzene And[1,2-b:4,5-b']dithiophene-2,6-diyl)bis(trimethyltin) (90.5mg, 0.10mmol) and catalyst tetrakistriphenylphosphine palladium (5.86mg , 0.005mmol) was added to a 10mL microwave tube, anhydrous xylene (2.5mL) was added to dissolve, and the silica gel cap was sealed. Place the microwave tube in the microwave reactor, set the temperature program: 80°C for 2 minutes, 120°C for 2 minutes, 160°C for 2 minutes, and finally 200°C for 45 minutes. In 200mL methanol, carry out Soxhlet extraction successively with methanol, acetone, n-hexane, dichloromethane and chloroform, then add the aqueous solution of sodium diethyldithiocarbamate trihydrate (225mg, 1mmol , 100mL water), stirred at 60°C for 8 hours, remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| energy conversion efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com