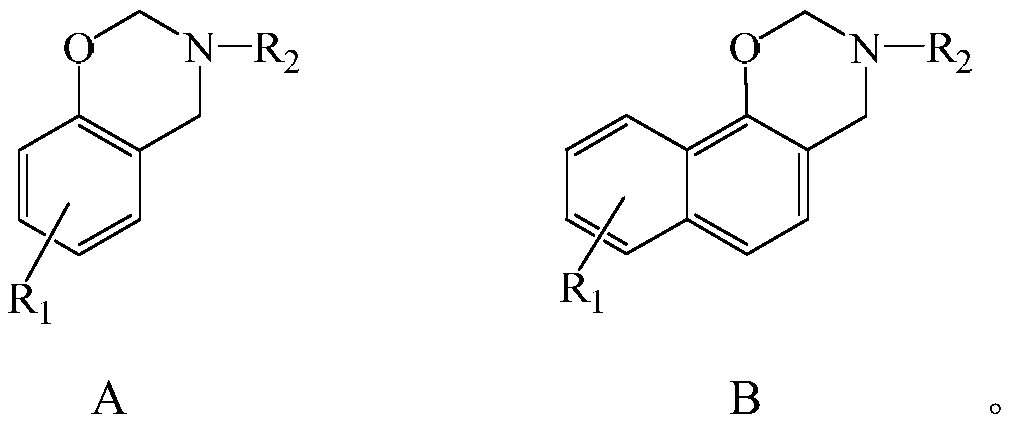
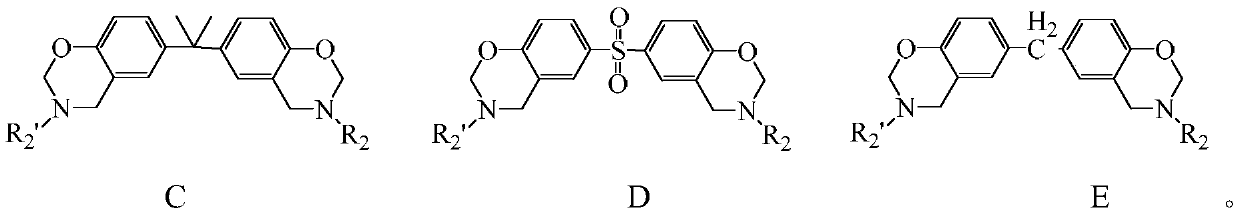
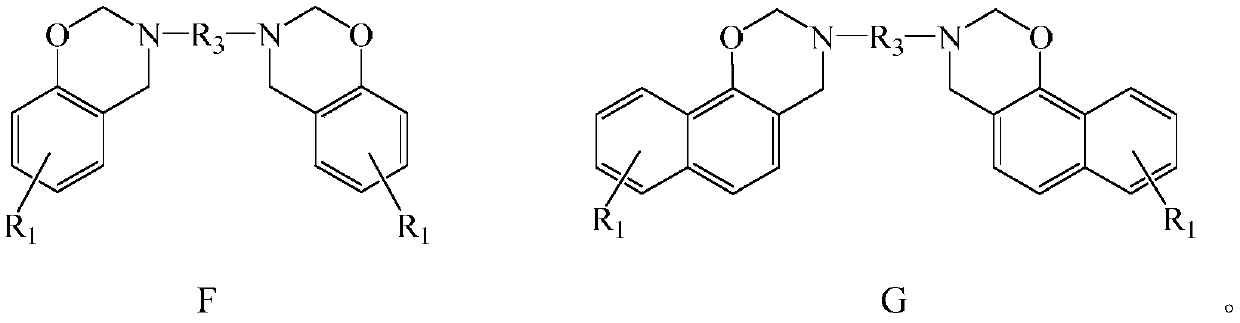
Halogen-free swelling flame-retardant system containing benzoxazine charcoal forming agent and flame-retardant thermoplastic resin of halogen-free swelling flame-retardant system
A thermoplastic resin and benzoxazine technology, applied in the field of polymer materials, can solve the problems of easy migration and precipitation, poor dispersion, poor compatibility of substrates, etc., achieve enhanced interaction, high flame retardant efficiency, and improve unfavorable effect of influence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The weight ratio of 69.9% POM, 20% ammonium polyphosphate, 6% melamine, 3% bisphenol A aniline benzoxazine and 1% phenolic benzoxazine, 0.1% nanometer montmorillonite and a small amount of antioxidant 1010 Premix on a high-speed mixer for 10 minutes, then melt, extrude and granulate in a twin-screw extruder at 165-175°C, and inject into molding on an injection molding machine.
[0043] Wherein the preparation method structural formula of bisphenol A aniline type benzoxazine used is identical with comparative example 1, and the preparation method of phenol type benzoxazine is similar to the preparation method of bisphenol A aniline type benzoxazine in comparative example 1, It is synthesized from 4-cyanophenol, 3-aminopropyltriethoxysilane and paraformaldehyde at a molar ratio of 1:1:2, and its structural formula is as follows:
[0044]
[0045] The limiting oxygen index of the obtained halogen-free intumescent flame-retardant polyoxymethylene is 41.5%, and the vertic...
Embodiment 2
[0047] The weight ratio of 69.7% POM, 20% ammonium polyphosphate, 6% melamine, 2% bisphenol A aniline benzoxazine and 2% bisphenol F aniline benzoxazine, 0.3% graphene oxide and a small amount of antioxidant Agent 1010 was pre-mixed on a high-speed mixer for 10 minutes, then melted and extruded in a twin-screw extruder at 165-175°C to granulate, and then injection-molded on an injection molding machine.
[0048] Wherein the structural formula of bisphenol A aniline benzoxazine used is the same as that of comparative example 1, and the preparation method of bisphenol F aniline benzoxazine is similar to that of bisphenol A aniline benzoxazine in comparative example 1 , synthesized by bisphenol F, aniline and paraformaldehyde in a molar ratio of 1:2:4, its structural formula is as follows:
[0049]
[0050] The limiting oxygen index of the obtained halogen-free intumescent flame-retardant polyoxymethylene is 49.7%, and the vertical burning is V-1 level.
Embodiment 3
[0052] Premix 72% POM, 20% ammonium polyphosphate, 6% melamine, 2% silane-containing bisphenol A type benzoxazine and a small amount of antioxidant 1010 on a high-speed mixer for 10 minutes, and then extrude on a twin-screw Melt and extrude at 165-175°C to granulate in the exiting machine, and then injection-molded on the injection molding machine.
[0053] The preparation method of bisphenol A type benzoxazine containing silane structure used is similar to the preparation method of bisphenol A aniline type benzoxazine in Comparative Example 1, by bisphenol A, 3-aminopropyltriethoxysilane and Paraformaldehyde is synthesized at a molar ratio of 1:2:4, and its structural formula is as follows:
[0054]
[0055] The limiting oxygen index of the obtained halogen-free intumescent flame-retardant polyoxymethylene is 52.1%, and the vertical burning is V-0 level.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com