Animal influenza antibody blocking test strip
A technology for detecting test strips and antibodies, applied to the field of poultry disease antibody detection devices, can solve the problems of census unsuitable for large-scale samples, high cost, time-consuming, etc. Easy and fast effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] (1) Preparation of influenza virus immune antigen
[0054] Inoculate 9-11-day-old SPF chicken embryos with different subtypes of influenza virus (H1N1, H3N2, H5N1, H7N9, H9N2) through the allantoic cavity, collect the allantoic fluid at 36-48 hours, and purify the virus antigen by differential centrifugation at 4°C 3000r / Centrifuge at low speed for 30 min to remove impurities, ultracentrifuge at 60,000 r / min at 4°C for 1 h, and resuspend the pellet with 7 mL of 0.01 mol / L PBS (pH 7.2). The HA titer of influenza virus was determined to be 2 by hemagglutination assay (HA). -12 above. The influenza virus was inactivated by adding 0.02% β-propiolactone (BPL) at a final concentration of 37° C. for 9 hours to the allantoic fluid of the influenza virus to prepare the inactivated virus.
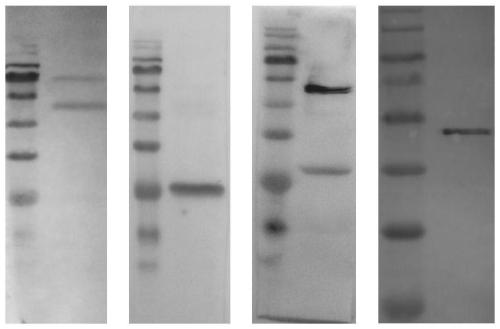
[0055] (2) Preparation of anti-influenza virus monoclonal antibody
[0056] (2.1) Establishment of hybridoma cell lines
[0057] Use the inactivated influenza virus or vaccine as the immu...
Embodiment 1
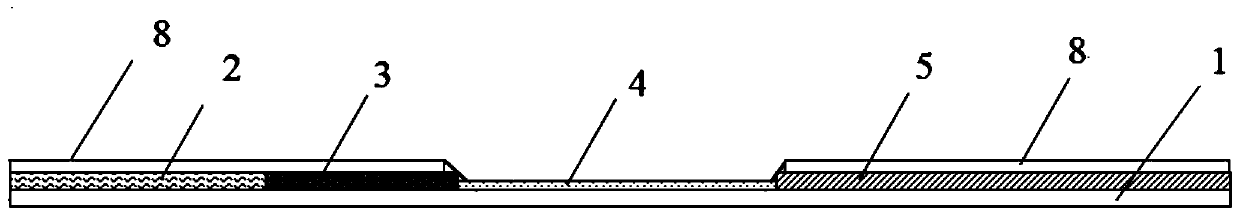
[0127] Example 1: Monitoring the level of maternal antibodies to animal influenza, collecting animal serum, taking 100 μL of serum samples and adding 300 μL of PBS or saline for dilution to prepare a serum solution to be tested, and using animal influenza antibody blocking detection test paper according to the operation method (16) Detection and result judgment, detection of animal influenza maternal antibody level. Two red bands (test line and quality control line) "||" appear on the test paper, and the color intensity of the test line T is equivalent to that of the blank control, which is negative for influenza antibodies, indicating that the serum sample to be tested does not contain influenza maternal antibodies; Two red bands "||" appeared, but the detection line T was significantly weaker than that of the blank control, which was weakly positive for influenza antibodies, indicating that the serum samples to be tested contained low levels of influenza maternal antibodies, ...
Embodiment 2
[0128] Example 2: Evaluation of the immune effect of animal influenza vaccines. 2 to 3 weeks after immunization with animal influenza inactivated vaccines, collect serum from immunized animals, take 100 μL of serum samples and add 300 μL of PBS or normal saline for 1:2, 1:4, 1:8 …Double ratio dilution, prepare the serum solution to be tested, use the animal influenza antibody blocking test paper to perform detection and result judgment according to the operation method (16), and detect the animal influenza immune antibody level. Two red bands (test line and quality control line) "||" appear on the test paper, and the color intensity of the test line T is equivalent to that of the blank control, which is negative for influenza antibodies, indicating that the serum sample to be tested does not contain influenza neutralizing antibodies; Two red bands "||" appeared, but the detection line T was significantly weaker than that of the blank control, which was weakly positive for influ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com