Chitosan-based amphiphilic self-assembling nano carrier and preparation method and application thereof
A nano-carrier and chitosan technology, applied in the direction of medical preparations with non-active ingredients, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of chitosan's unideal solubility and achieve good biological Safety, good dispersibility, and uniform particle size
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
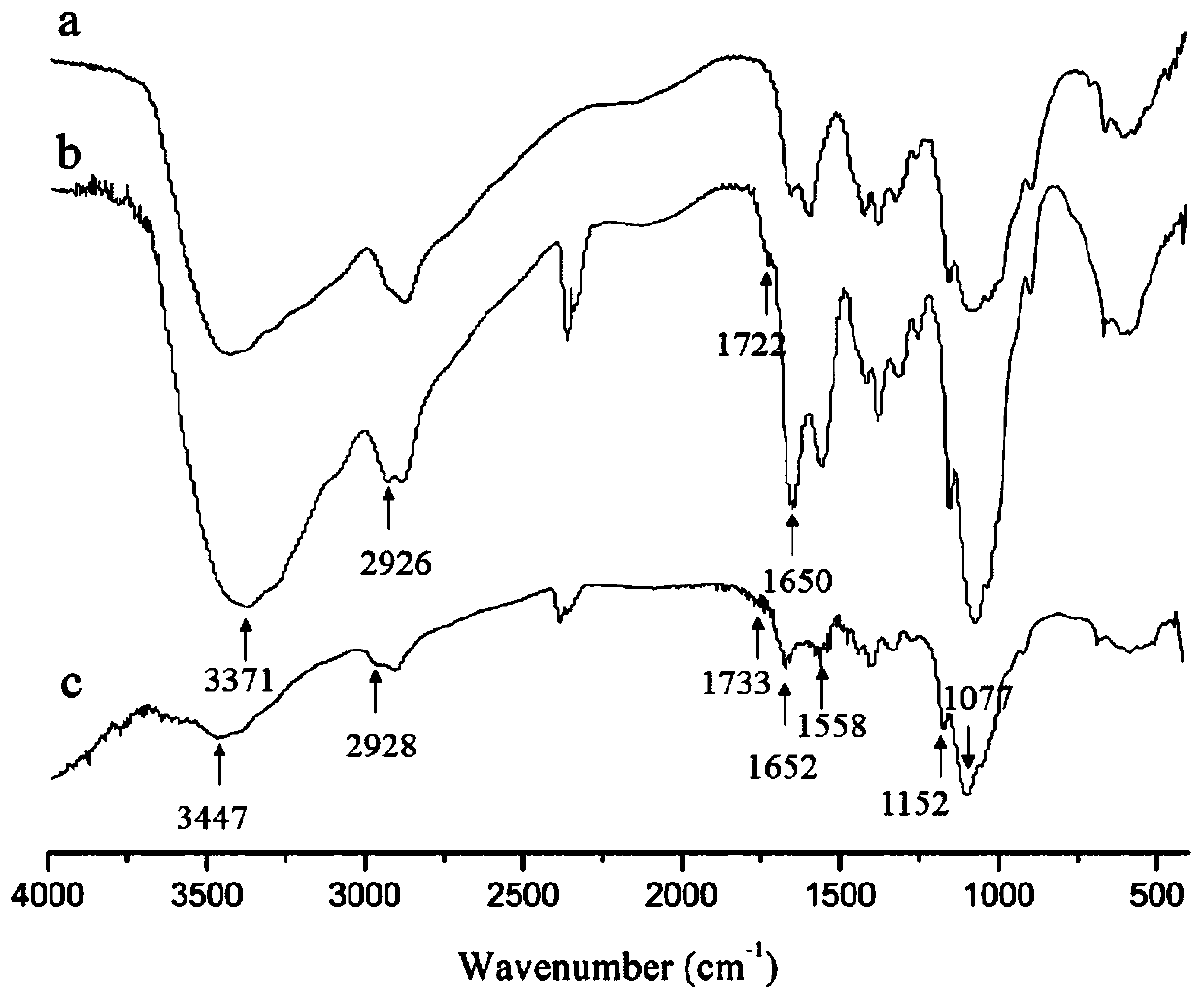
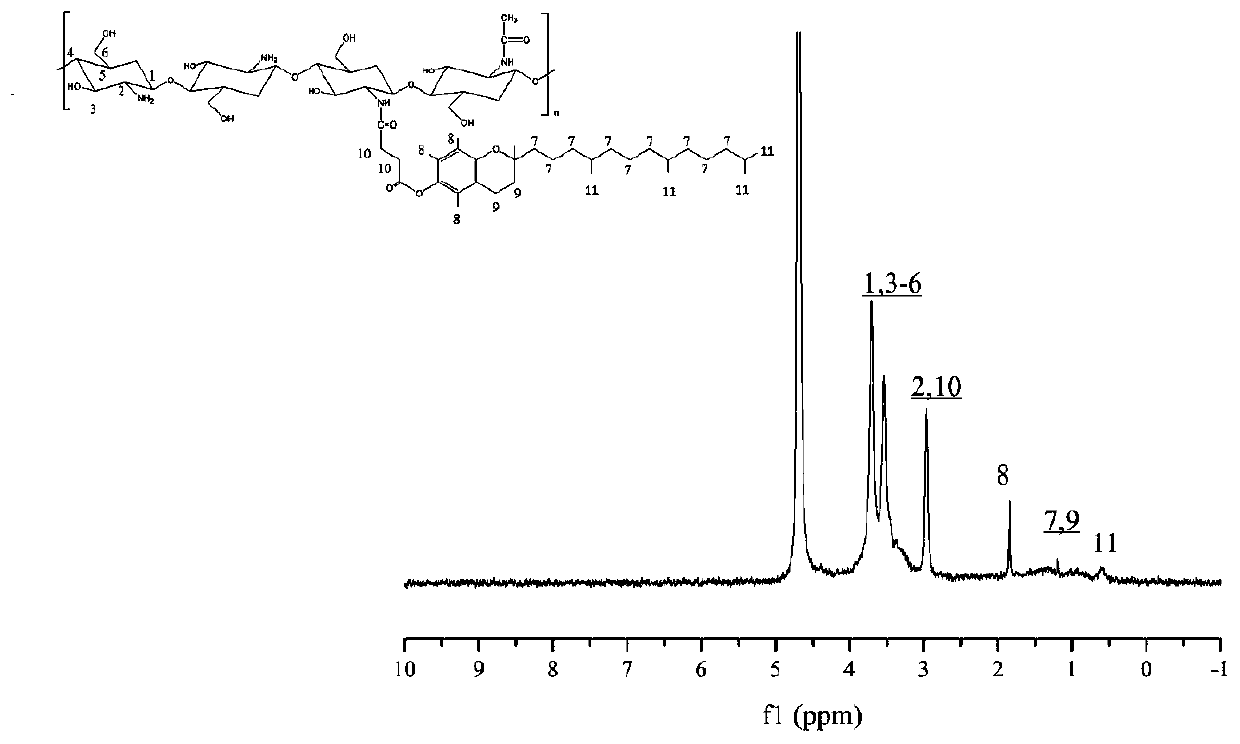
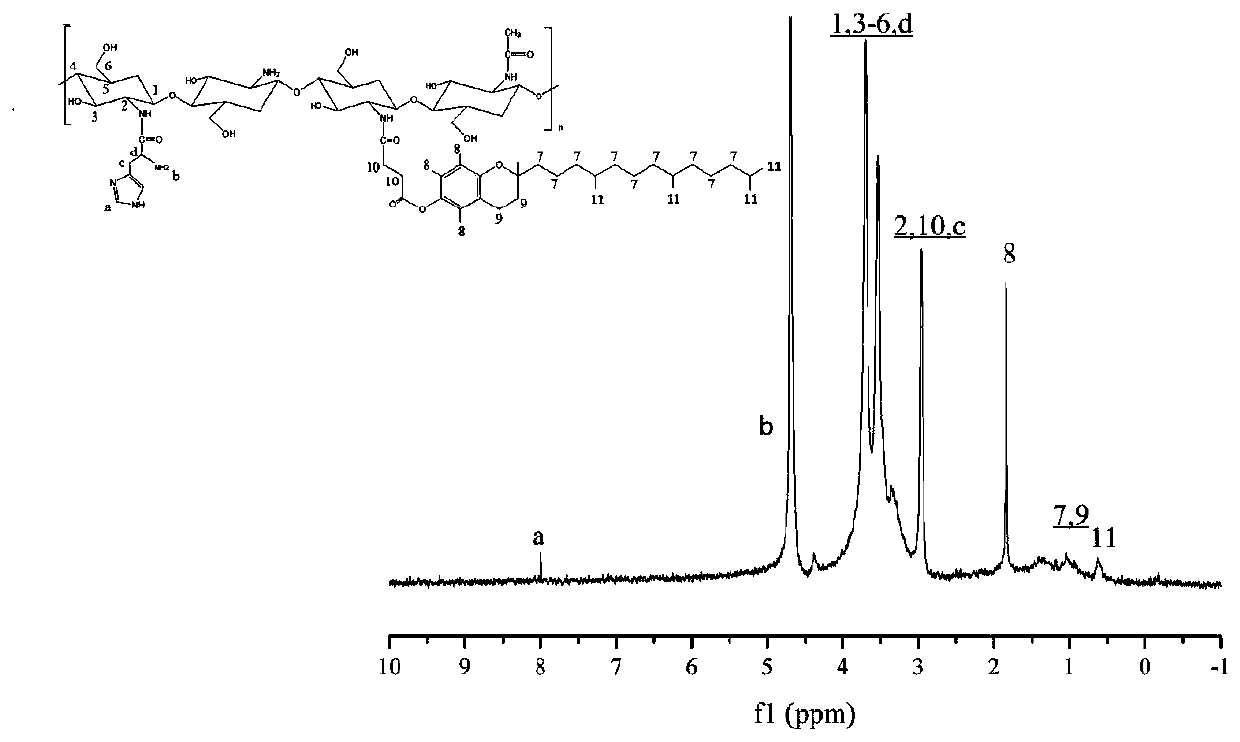
[0052] Accurately weigh 0.24g of low-molecular-weight chitosan and dissolve it in 30mL of 1% hydrochloric acid solution, stir rapidly with a glass rod for 10min at room temperature, and then dissolve it completely by magnetic stirring, and set aside. Accurately weigh 0.79g VES and place it in a three-necked flask, use a graduated cylinder to measure 40mL DMF and pour it into the three-necked flask, stir to dissolve it completely, then accurately weigh 0.32g EDC and 0.19g NHS and add them to the above solution, and stir magnetically for 2 hours , the dissolved chitosan solution was added dropwise into a three-neck flask through a constant pressure titration funnel at a rate of 1 drop / second, and mechanically stirred for 48 hours at room temperature. Pour the reacted mixed solution into 5 times the volume of absolute ethanol to precipitate, stir for 8 hours, centrifuge at room temperature at 8000rpm for 20 minutes, discard the supernatant, collect the precipitate, wash the precip...
Embodiment 2
[0056] Digest L929 cells in the logarithmic growth phase with 0.25% trypsin, add DMEM medium containing 10% FBS to stop the digestion, resuspend the cells, and dilute the concentration to 2×10 with DMEM medium after centrifugation 4 per mL, the diluted cells were planted in a 96-well plate, with 200 μL of cell suspension per well. After the 96-well plate was placed in the cell incubator for 24 h, the culture medium in the cell plate was discarded, and 200 μL of fresh medium containing VCH blank nanoparticles with different concentrations was added, and the concentration of VCH blank nanoparticles was set to 10, 50, 100, 200 μg / mL, and the cells cultured in normal DMEM medium were set as the control group, and the cells only containing DMEM medium without planting were set as the blank control group, and 6 replicates were set for each group. Gently shake, culture in the cell incubator for 24 and 48 hours, observe the cell morphology under an inverted microscope and take picture...
Embodiment 3
[0062] 24 KM male mice were randomly divided into 2 groups, the experimental group and the control group, 12 in each group, and the experimental group injected 0.2mL VCH nanoparticles into the mice every day according to the dose of 10mg / Kg through the tail vein injection method. Saline solution, the control group injected 0.2mL physiological salt injection into the tail vein of the mice every day for seven consecutive days, and observed the daily activity status of the mice and whether there were symptoms of poisoning reaction. After the end of the administration, on the eighth day, blood was taken from each mouse by taking blood from the eye socket, and the blood taken out was collected separately to contain sodium citrate and EDTAK 2 The four blood coagulation items and blood routine tests were carried out in the blood vessels; the mice were sacrificed after the blood collection was completed, the heart, liver, spleen, lung and kidney were taken out, the fascia was peeled of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com