A drug carrier system capable of targeted drug delivery in cancer cells and preparation method thereof
A technology of targeted drug delivery and carrier system, which is applied in the field of drug carrier system with the ability of targeted drug delivery in cancer cells and its preparation, which can solve hypoxia, reduce the effective oxygen concentration in PDT, and the imbalance between oxygen supply and oxygen consumption And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
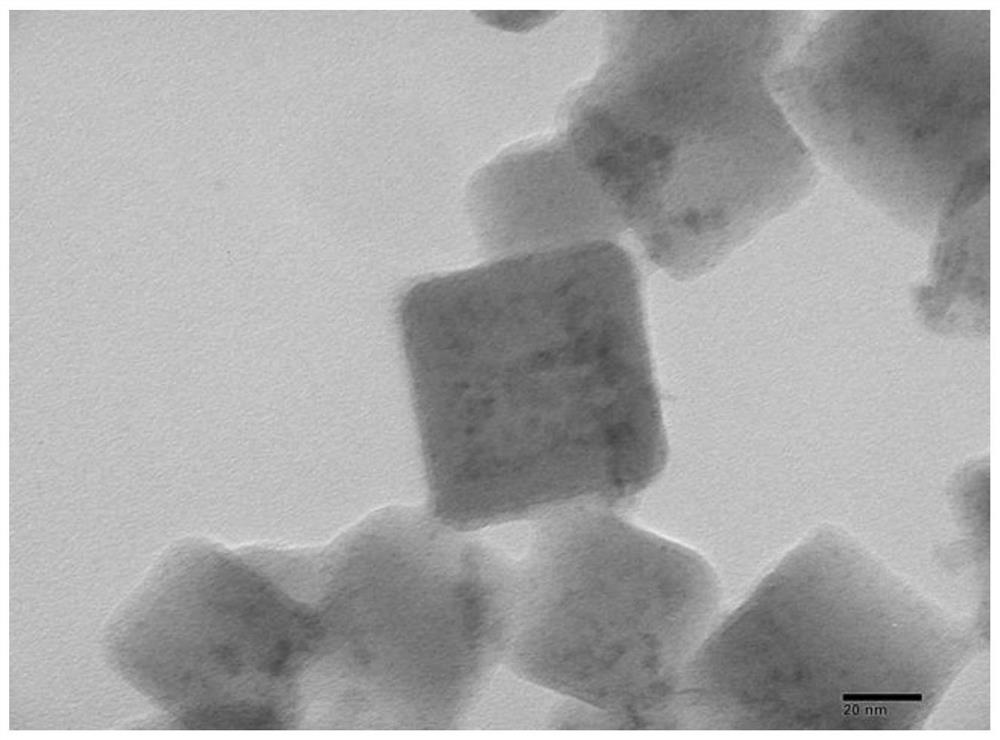
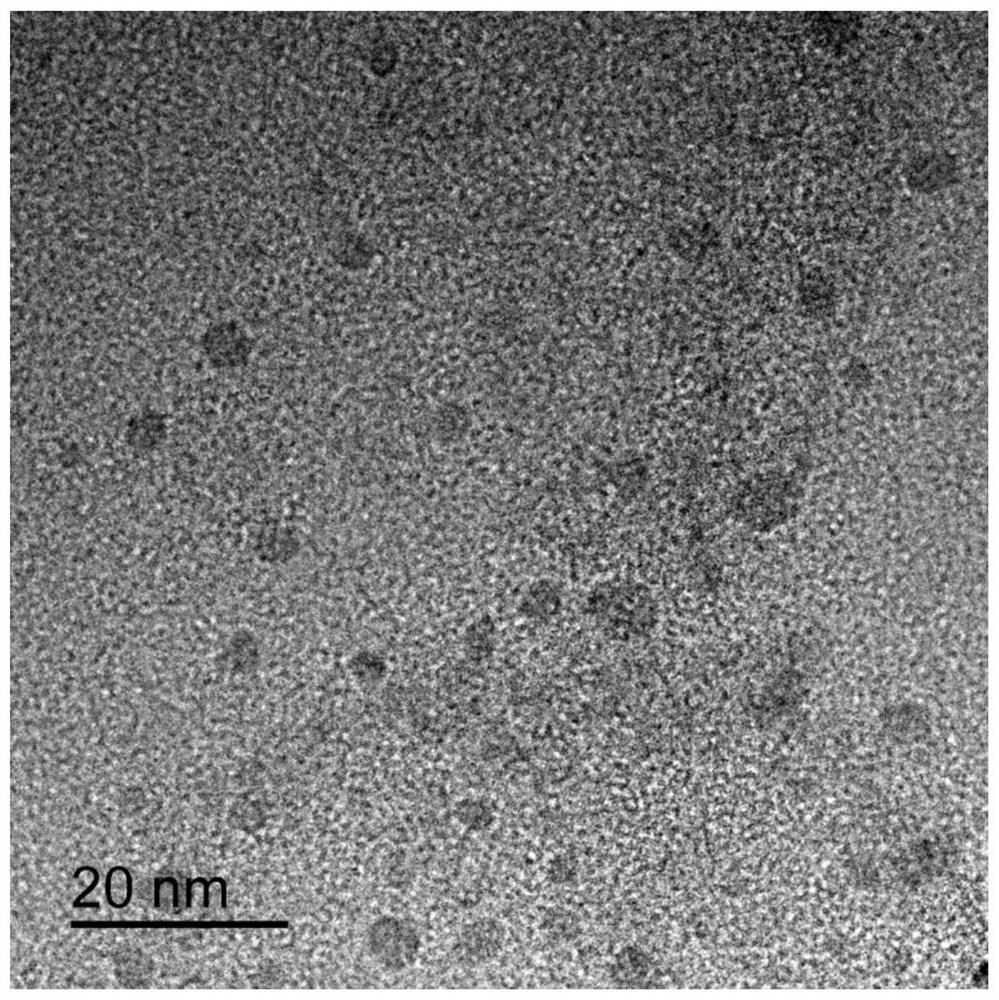
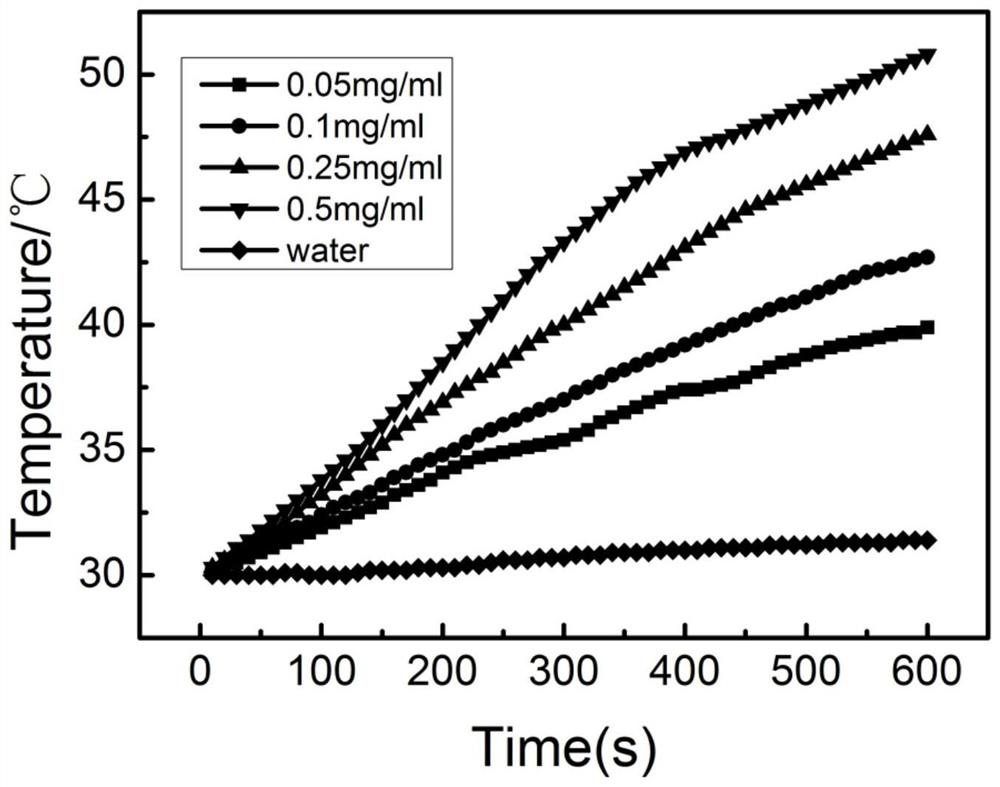
Image
Examples
Embodiment 1
[0065] A method of preparing a composite nano drug carrier system, including the steps of:
[0066] Synthetic PB-CD-NH 2
[0067] The PB 200 mg was scattered in PBS of 100 mL of pH of 5.5, and then the ice water bath was 4 ° C, EDC (1- (3-dimethylaminopropyl) -3-ethyl carbide hydrochloride 2.87 G and N-hydroxy succinimide 1.7262 g of reaction for 24 hours; then adding 140 mg-β-Cd and 60 mg of cystylene metalline salts for 24 hours, centrifugation, washed the resulting solid product, which is PB-CD-NH. 2 .
[0068] 2. Synthesis PB-C-DOTS-CD
[0069] The carbon quantum point 60 mg was scattered in PBS of 100 mL of a pH of 5.5, and then EDC1.7262G and N-hydroxy succinimide were added at 4 ° C for 24 hours, and then 200 mg Pb-CD-NH2 was added. The reaction was cleaned at room temperature for 24 h, and the resulting solid product was washed, which was PB-C-DOTS-CD.
[0070] 3. Synthesize PLL (NF)
[0071] 200 mg of PLL, 100 mg of potassium carbonate and 5-chloro-2-nithenyl trifluorome...
Embodiment 2
[0089] A method of preparing a composite nano drug carrier system, including the steps of:
[0090] Synthetic PB-CD-NH 2
[0091] It is weighing PB 200 mg to disperse in 100 ml of deionized water, and pH is adjusted to 5-6 with hydrochloric acid (if it is adjusted below 5, a small amount of sodium hydroxide is adjusted), and then the ice water bath is 4 ° C, add EDC (1- (1-) 3-dimethylaminopropyl) -3-ethyl carbon diimide hydrochloride 2.87 g of N-hydroxy succinimide 1.7262 g, reaction 24 hours; then add 140 mgeda-β-Cd and 60 mg cysteamine salts The acid salt reaction was 24 hours, and the obtained solid product was centrifuged, that is, PB-CD-NH2.
[0092] 2. Synthesis PB-C-DOTS-CD
[0093] The carbon quantum dot was scattered in water, and pH was adjusted to 5-6 with hydrochloric acid, and then EDC 1.7262 g and N-hydroxy succinimide was added to 2.87 g for 24 hours, and then 200 mg Pb-Cd was added. -NH2 room temperature reaction 24 h, centrifugal washed solid product, which is P...
Embodiment 3
[0101] A method of preparing a composite nano drug carrier system, including the steps of:
[0102] Synthetic PB-CD-NH 2
[0103] The PB 200 mg was scattered in PBS of 100 mL of pH of 5.5, and then the ice water bath was 4 ° C, EDC (1- (3-dimethylaminopropyl) -3-ethyl carbide hydrochloride 2.87 G and N-hydroxy succinimide 1.7262 g, reaction for 24 hours; then adding 180 mGEDA-β-CD and 20 mg of cystamine dihydrochloride for 24 hours, centrifugation, washed the resulting solid product, which is PB-CD-NH. 2 .
[0104] 2. Synthesis PB-C-DOTS-CD
[0105] The carbon quantum point 60 mg was scattered in PBS of 100 mL of a pH of 5.5, and then EDC1.7262G and N-hydroxy succinimide were added at 4 ° C for 24 hours, and then 200 mg Pb-CD-NH2 was added. The reaction was cleaned at room temperature for 24 h, and the resulting solid product was washed, which was PB-C-DOTS-CD.
[0106] 3. Synthesize PLL (NF)
[0107] It is weighed 200 mg PLL, 100 mg of potassium carbonate and 5-chloro-2-nithenyl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com