A kind of preparation method of multistage sustained-release drug-loaded nano-short fibers
A drug-loaded nano and short fiber technology, which is applied in fiber treatment, pharmaceutical formulations, medical preparations of non-active ingredients, etc., can solve the problems of reducing the utilization rate of drugs, secondary harm to patients, etc., and overcome multi-drug resistance , low cost, and the effect of improving the treatment effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] (1) 830.8mg Mg(NO 3 ) 2 ·6H 2 O and 603.8mg Al(NO 3 ) 3 9H 2 O was dissolved in 50 mL of ultrapure water, and stirred vigorously at 25 °C for 20 min to fully dissolve the two compounds. Mix the two solutions well. Nitrogen was flushed to catch oxygen 5 times, and then 15 mL of 1M NaOH solution was added in a nitrogen atmosphere to adjust the pH of the solution to 9.5. Stir vigorously at 25°C for 48 h to fully react. Collect the reacted solution in a centrifuge tube, set the rotation speed at 6000 rpm, and centrifuge for 10 minutes, and the white precipitate obtained is LDH. Add an appropriate amount of ultrapure water and use a vortex shaker to disperse the precipitate evenly, perform ultrasonic washing, and then set the rotation speed at 6000rpm for 10 minutes. Repeat the operation 3 times to obtain pure LDH precipitate. LDH nanoparticles were obtained by vacuum freeze-drying.
[0045] (2) Fully dissolve 20.3 mg DOX and 10.3 mg of LDH prepared in step (1) in 10...
Embodiment 2
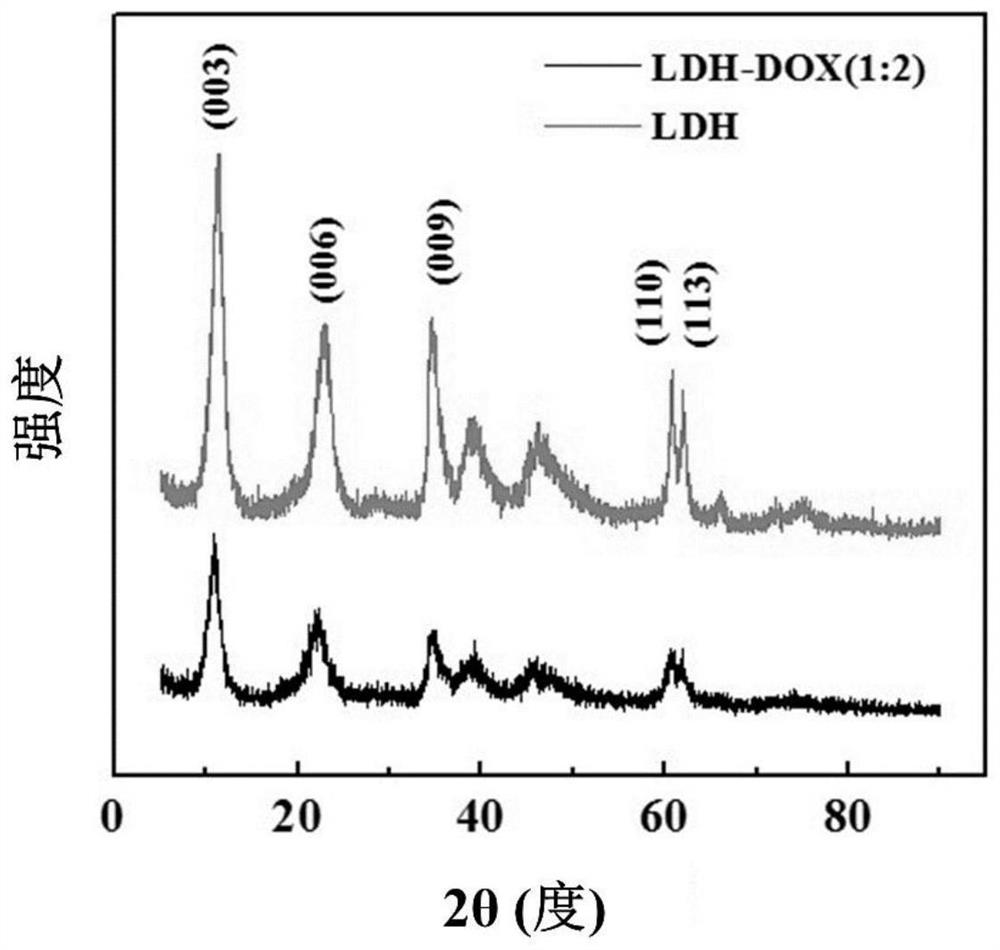
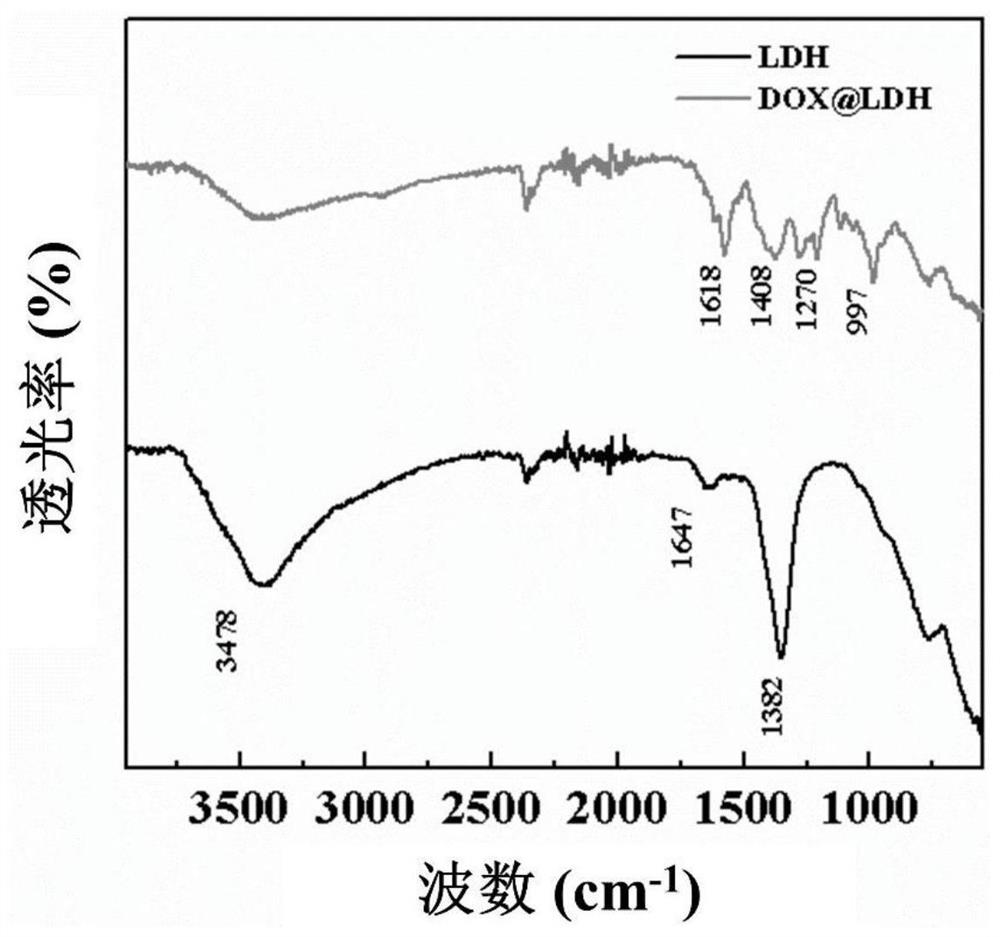
[0049] The present invention uses scanning electron microscope (SEM), transmission electron microscope (TEM), X-ray diffraction (XRD), infrared spectrum analysis (FTIR), ultraviolet-spectrophotometer (UV-Vis), coagulation experiment, cell viability analysis ( CCK-8 test), laser confocal microscopy to characterize the morphology of LDH prepared by the present invention and multi-level slow-release drug-loaded nano-short fibers, the long-term multi-level drug release effect of short fibers and the effect of short fibers in drug-resistant cancer cells application potential.
[0050] Scanning Electron Microscopy Test:
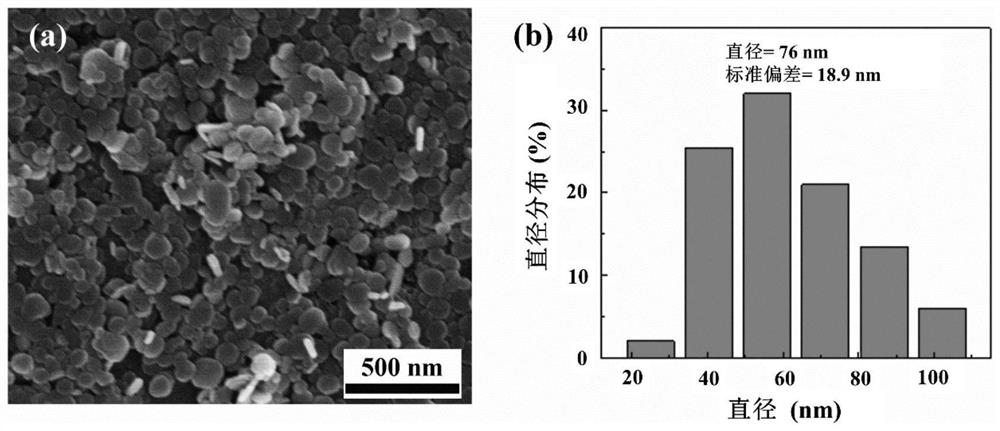
[0051] The LDH obtained in step (1) of Example 1 and the DOX@LDH / α-TOS / PLGA short fiber obtained in step (5) were characterized by a scanning electron microscope. The SEM results of LDH are as follows figure 2 As shown in a-b, the diameter of LDH is 76±18.9nm, and it is relatively uniform hexagonal or disc-shaped. The SEM results of DOX@LDH / α-TOS / PLGA short fib...
Embodiment 3
[0059] In vitro release kinetics test
[0060] Using UV-spectrophotometer (UV-Vis) to measure drug release from short fibers in buffer solution with different pH at different time points. Add 5 mg of DOX / PLGA, α-TOS / PLGA and DOX@LDH / α-TOS / PLGA short fibers into 1 mL of PBS buffer solution with pH=7.4, 6.8 and 5.5, and ultrasonically disperse evenly, and put them into a dialyzer with a molecular weight cut-off of 5000 In the bag, put the dialysis bag filled with materials into a 50mL centrifuge tube, add 9mL of PBS buffer solution corresponding to the pH, and put it in a constant temperature shaker for drug sustained release experiments. Take the solution at 1, 2, 4, 8, 16, 24, 48, and 72 hours in the first 3 days, take the solution every two days from the 4th day to the 20th day, and take the solution every five days from the 20th day to the 60th day . Take 1 mL each time, and then add 1 mL of PBS buffer solution corresponding to the pH to the centrifuge tube. Measure the a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com