Compound drug coated balloon catheter and preparation method thereof
A drug coating and balloon catheter technology, applied in balloon catheters, catheters, coatings, etc., can solve the problems of drug balloon inhibition of restenosis and short drug release cycle, and achieve less drug loss, inhibition of proliferation, The effect of reducing toxic side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
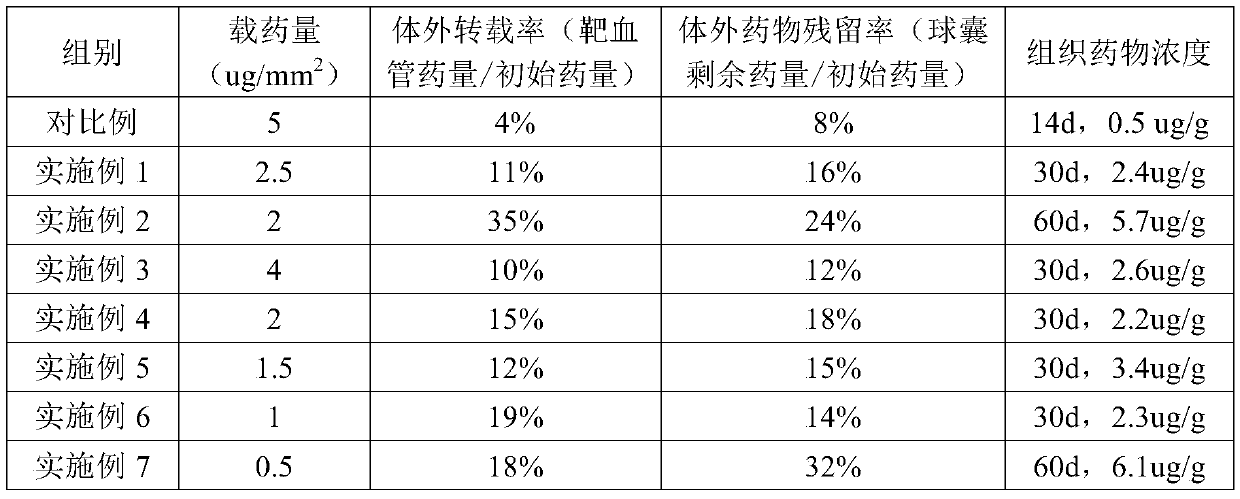
Image
Examples
Embodiment 1
[0063] 1. Raw materials:
[0064] 1. Drugs: Paclitaxel;
[0065] 2. Low molecular weight carrier: mannitol, molecular weight 182;
[0066] 3. High molecular weight carrier: PEG600, molecular weight 600;
[0067] 4. Purified water, medical grade ethanol.
[0068] 2. Preparation method
[0069] 1. Prepare an ethanol solution of high molecular weight carrier PEG600 and paclitaxel at a concentration of 21 mg / ml, wherein the mass ratio of paclitaxel and PEG600 is 20:1, and mix thoroughly to obtain solution 1;
[0070] 2. Prepare an aqueous solution of low-molecular-weight carrier mannitol with a concentration of 10 mg / ml, and mix thoroughly to obtain solution 2;
[0071] 3. Mix 10ml of solution 1 and 1ml of solution 2 to obtain a coating solution, wherein the mass ratio of paclitaxel to high molecular weight carrier to low molecular weight carrier is 20:1:1;
[0072] 4. Spray the coating solution onto the outer surface of the balloon by ultrasonic atomization;
[0073] 5. Aft...
Embodiment 2
[0075] 1. Raw materials:
[0076] 1. Drug: rapamycin;
[0077] 2. Low molecular weight carrier: phosphatidylcholine, molecular weight 759;
[0078] 3. High molecular weight carrier: polylactic acid-glycolic acid copolymer (PLGA), molecular weight 20000;
[0079] 4. Purified water, medical grade ethyl acetate, medical grade ethanol.
[0080] 2. Preparation method
[0081] 1. Dissolve the high molecular weight carrier PLGA and rapamycin in ethyl acetate at a concentration of 20 mg / ml, wherein the mass ratio of rapamycin to PLGA is 1:1, and mix thoroughly to obtain solution 3;
[0082] 2. Dissolve the low-molecular-weight carrier phosphatidylcholine in ethanol at a concentration of 10 mg / ml, and mix thoroughly to obtain solution 4;
[0083] 3. Dissolve polyvinyl alcohol in water to obtain 1 mg / ml polyvinyl alcohol solution 5;
[0084] 4. Spray the solution 3 into the high-speed stirring solution 5 by ultrasonic atomization, continue to stir for 2 hours, and then filter, wash...
Embodiment 3
[0089] 1. Raw materials:
[0090] 1. Drug: zotarolimus;
[0091] 2. Low molecular weight carrier: PEG400, molecular weight 400;
[0092] 3. High molecular weight carrier: poloxamer, molecular weight 3000;
[0093] 4. Medical grade ethanol.
[0094] 2. Preparation method
[0095] 1. Prepare an ethanol solution of high molecular weight carrier poloxamer and zotarolimus, the concentration is 12 mg / ml, wherein the mass ratio of zotarolimus to poloxamer is 1:5, and mix well to obtain solution 1;
[0096]2. Prepare an ethanol solution of low molecular weight carrier PEG400 at a concentration of 10 mg / ml, and mix thoroughly to obtain solution 2;
[0097] 3. Mix 10ml of solution 1 and 5ml of solution 2 to obtain a coating solution, wherein the mass ratio of zotarolimus to high molecular weight carrier to low molecular weight carrier is 2:10:5;
[0098] 4. Spray the coating solution onto the outer surface of the balloon by ultrasonic atomization;
[0099] 5. After the coating is ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com