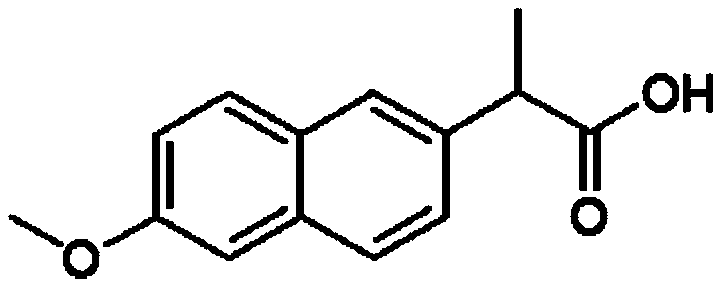
D, L-naproxen one-pot synthesis method
A synthesis method and technology of naproxen, applied in chemical instruments and methods, preparation of oxygenated compounds, preparation of carbon-based compounds, etc., can solve the problems of low reaction efficiency, long reaction time, large amount of waste water, etc., and improve the reaction efficiency , The effect of shortening the reaction time and reducing waste water discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
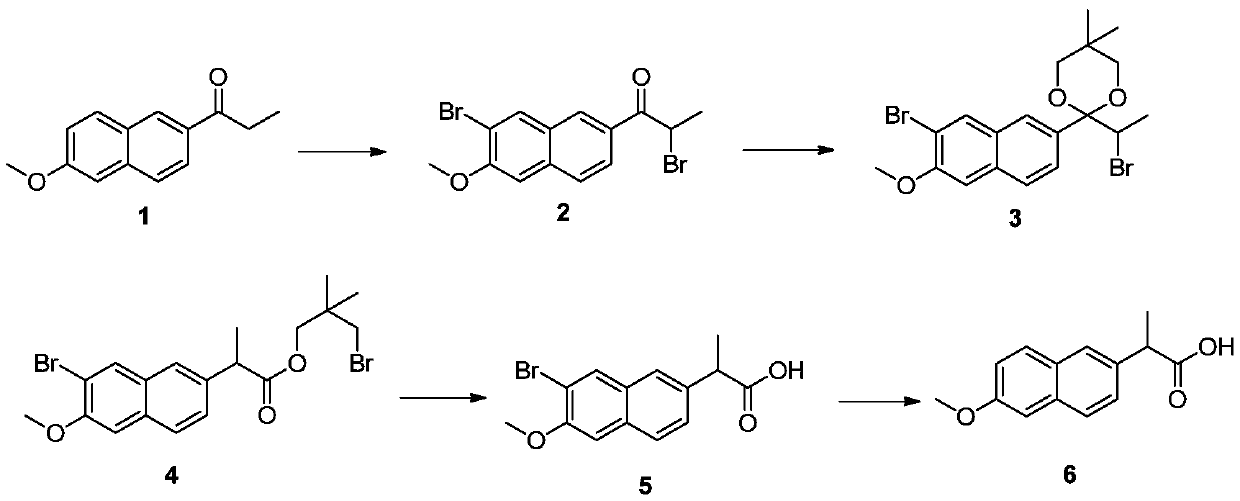
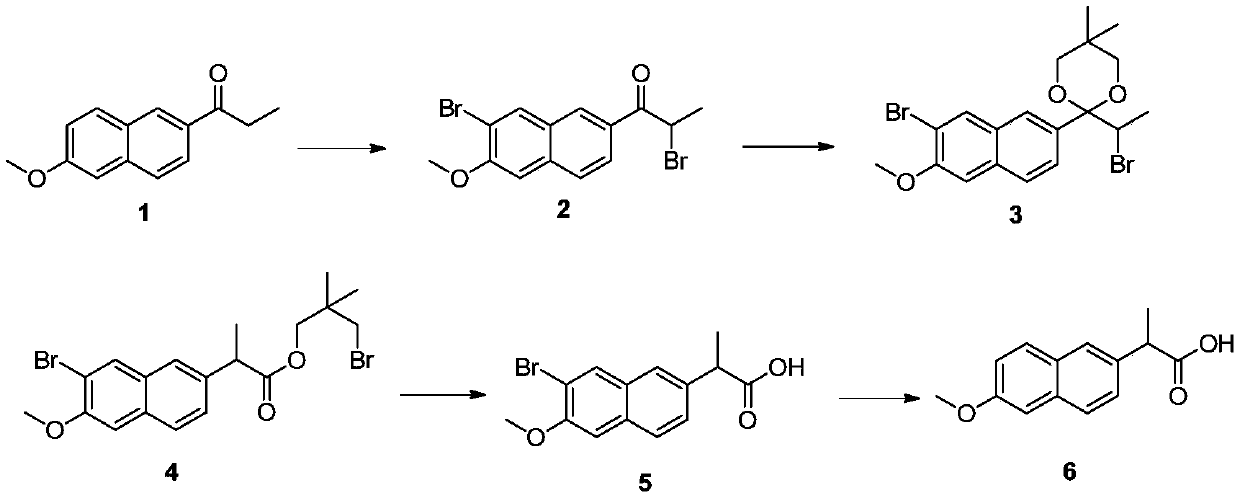
[0032] Put 1.5 kg of xylene and 600 g (1.59 mol) of phenyltrimethylammonium tribromide into a 5 L three-necked flask, stir and dissolve at 35°C-45°C for half an hour. Then cool down to 15° C., add 320 g (1.5 mol) of 6-methoxy-2-propionylnaphthalene, continue the reaction at this temperature for 8 hours, add 1.5 kg of water to the system and stir to separate the liquid. The upper organic phase was directly used in the next reaction without further purification. The aqueous phase is collected for recovery of phenyltrimethylammonium tribromide.
[0033] Pour the xylene solution from the previous step into a 3-liter three-necked flask and heat to boiling. After azeotropic removal of water in the system, cool down to 60°C and add 115 grams (1.1 mol) of neopentyl glycol and 40 grams (0.23 mol) of p-toluenesulfonic acid. . After the addition, the temperature was raised to reflux, the reflux was divided into water and reacted for 4 hours, the raw materials disappeared as...
Embodiment 2
[0038] Put 1.5 kg of xylene and 596 g (1.58 mol) of phenyltrimethylammonium tribromide into a 5 L three-necked flask, stir and dissolve at 35°C-45°C for half an hour. Then cool down to 25° C., add 320 g (1.5 mol) of 6-methoxy-2-propionylnaphthalene, continue the reaction at this temperature for 7 hours, add 1.5 kg of water to the system and stir to separate the liquid. The upper organic phase was directly used in the next reaction without further purification.
[0039] Pour the xylene solution from the previous step into a 3-liter three-necked flask and heat to boiling. After azeotropic removal of water in the system, cool down to 80°C and add 125 grams (1.2 mol) of neopentyl glycol and 78 grams (0.45 mol) of p-toluenesulfonic acid. . After the addition, heat up to reflux, reflux and divide water for 3 hours, HPLC detects that the raw material disappears, cool down to 80°C, set aside, and proceed to the next step.
[0040] Add 0.15 mol of zinc acetate to the xylene solution ...
Embodiment 3
[0044]Put 1.5 kg of xylene and 679 g (1.8 mol) of phenyltrimethylammonium tribromide into a 5 L three-necked flask, stir and dissolve at 35°C-45°C for half an hour. Then cool down to 18° C., add 320 g (1.5 mol) of 6-methoxy-2-propionylnaphthalene, continue the reaction at this temperature for 7 hours, add 1.5 kg of water to the system and stir to separate the liquid. The upper organic phase was directly used in the next reaction without further purification.
[0045] Pour the xylene solution from the previous step into a 3-liter three-necked flask and heat to boiling. After azeotropic removal of water in the system, cool down to 70°C and add 94 grams (0.9 mol) of neopentyl glycol and 26 grams (0.15 mol) of p-toluenesulfonic acid. . After the addition, heat up to reflux, reflux and divide water for 4 hours, HPLC detects that the raw material disappears, cool down to 70°C, set aside, and proceed to the next step.
[0046] Add 24 grams (0.3 mol) of zinc oxide to the xylene solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com