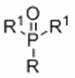
A kind of preparation method of alkyl phosphonate
A technology of alkyl phosphonylate and alkyl carboxylic acid, which is applied in the field of preparation of organic compounds, can solve the problems of diphenyl phosphine chloride being active, harsh reaction conditions, difficult to store, etc., and achieves easy-to-obtain raw materials and short reaction time , low toxicity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Example 1: Synthesis of n-pentyl diphenyloxate
[0055]
[0056] With n-hexoic acid, diphenolate as raw materials, the reaction steps are as follows:
[0057] Nimheracanoic acid (116 mg, 1 mmol), 4-dimethylaminopyridine (DMAP, 18.3 mg, 0.15 mmol), dihydrazapium diamine (DCC, 340 mg, 1.65 mmol), and hydrogenated (DCC, 340 mg, 1.65 mmol) 57 μ L, 1.9 mmol) and dichloromethane (5 mL), 0 o C is stirred for 2 hours, filtered, distilled off the solvent concentrate in the filtrate;
[0058] A concentrate was added to the concentrate (8 mg, 0.05 mmol), 2,2'-bipyridine (7.8 mmol), dichloroethane (1 mL) and diphenoxate (50.5 mg, 0.25 mmol) , Stirring at room temperature to the end of the reaction (TLC monitoring);
[0059] The crude product obtained after the reaction was separated (petroleum ether: acetone = 4: 1) to obtain a target product (yield 80%). The analysis data of the product is as follows: 1 H NMR (400 MHz, CDCL 3 ) δ 7.77-7.67 (m, 4H), 7.54-7.40 (m, 6H), 2.23 (m, ...
Embodiment 2
[0060] Example 2: Synthesis of n-butyl diphenyloxate
[0061] As a raw material for n -rete acid, diphenyl oxoslate as a raw material, the reaction steps are as follows:
[0062] Normalrene (102 mg, 1 mmol), 4-dimethylaminopyridine (DMAP, 18.3 mg, 0.15 mmol), dicyclohexyl carbonimide (DCC, 340 mg, 1.65 mmol), hydrogenated water (57 μ L, 1.9 mmol) and dichloromethane (5 mL), 10 o C is stirred for 2 hours, filtered, distilled off the solvent concentrate in the filtrate;
[0063] Add tetracetile copper (12.4 mg, 0.01 mmol), 4,4'-dibromo-2,2'-bipyridine (3.1 mg, 0.01 mmol), ethanol (1 ml) and diphenne (1 mL) and diphenne Surries (40.4 mg, 0.2 mmol), at room temperature, TLC monitoring to the end of the reaction;
[0064] The crude product obtained after the reaction was separated (petroleum ether: acetone = 4: 1) to obtain a target product (yield 82%). The analysis data of the product is as follows: 1 H NMR (400 MHz, CDCL 3 ) δ 7.77-7.67 (m, 4H), 7.54-7.40 (m, 6H), 2.23 (m, 2H),...
Embodiment 3
[0065] Example 3: Synthesis of n-hexyl diphenyl oxide
[0066] With nichelic acid, diphenyl oxoslate as a raw material, the reaction steps are as follows:
[0067] Normalikathic acid (130 mg, 1 mmol), 4-dimethylaminopyridine (DMAP, 18.3 mg, 0.15 mmol), dicyclohexyl carbonimide (DCC, 340 mg, 1.65 mmol), hydrogenated water (57 μ L, 1.9 mmol) and dichloromethane (5 mL), 20 o C is stirred for 2 hours, filtered, distilled off the solvent concentrate in the filtrate;
[0068] The copper (5.7 mg, 0.03 mmol), 5-bromo-2,2'-birane (7.1 mg, 0.03 mmol), acetonitrile (1 mL) and diphenyl oxine (1 mL) and diphenyl oxine ( 60.6 mg, 0.3 mmol), the mixing reaction reaction, TLC was monitored to the end of the reaction;
[0069] The crude product obtained after the completion of the reaction was separated by column chromatography (petroleum ether: acetone = 4: 1) to obtain a target product (yield 84%). The analysis data of the product is as follows: 1 H NMR (400 MHz, CDCL 3 ) δ 7.77-7.68 (m, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com