Preparation method of amino-substituted quinoline nitrogen heterocyclic ring
A nitrogen and flange technology is applied in the field of synthesis of a pharmaceutical intermediate 3-bromo-8-aminoquinoline, which can solve the problems of complete removal of reaction products, inability to promote, and damage to the whole bottle.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
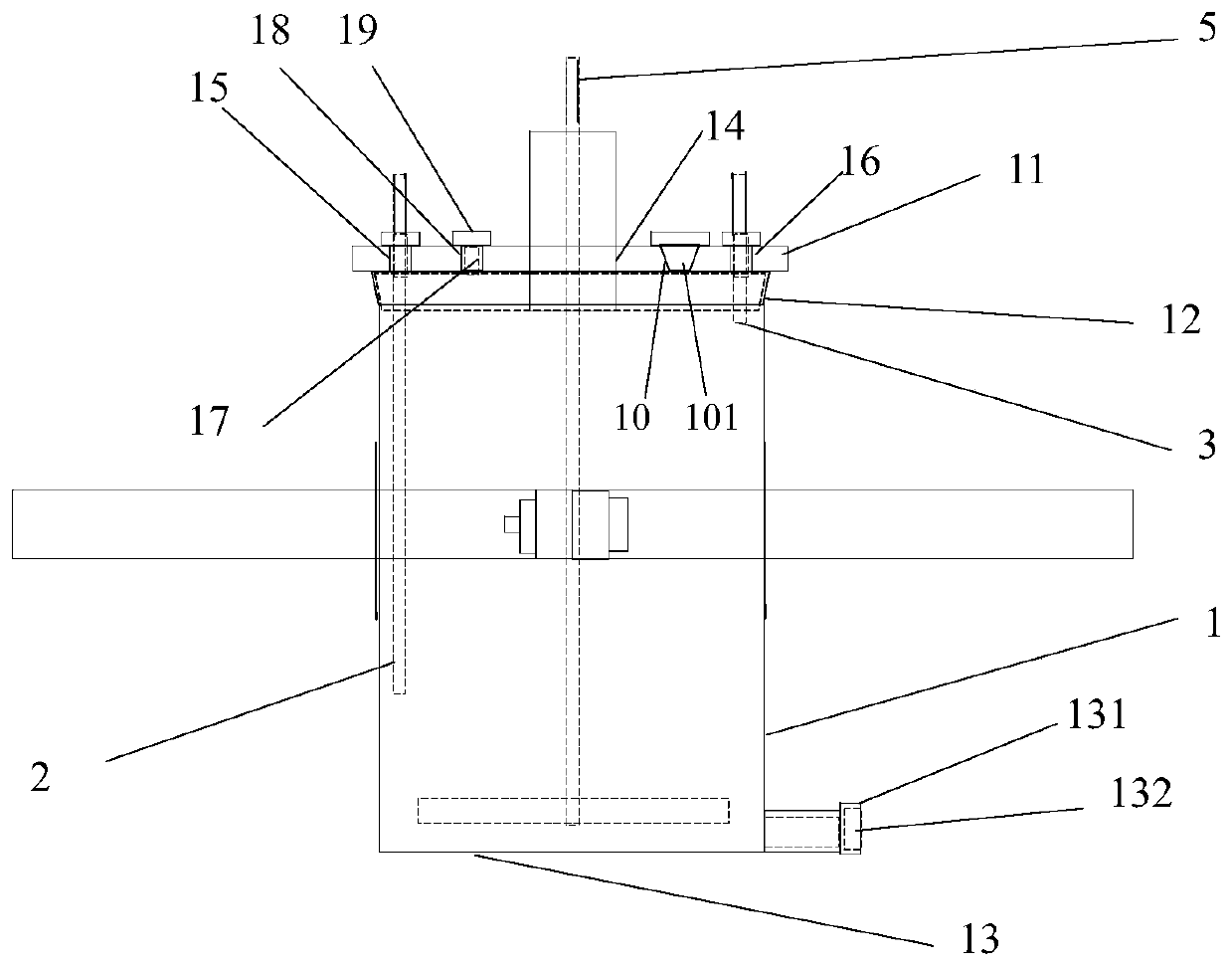
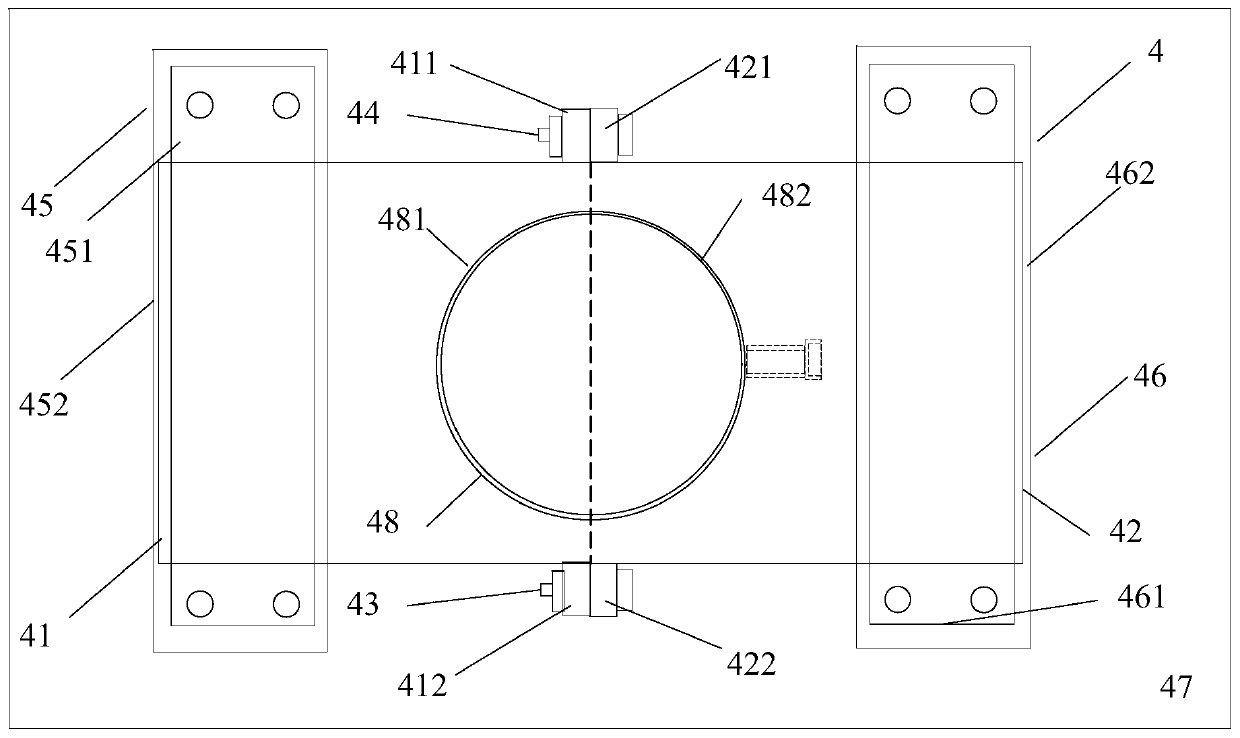
[0039] A reaction kettle, which has a body 1 made of glass, the body is equipped with a kettle cover 11, a lower flange 12, and a cylinder body 13. It is characterized in that: the reaction kettle also includes an air inlet combination 2, an air outlet combination 3, and a fixing device 4. Stirring section 5. The reaction kettle has improved the overall problems of the current four-neck bottle system as a whole: it is not stable enough, the bottom contact area is small, the heating and cooling are slow, the stirring speed cannot be increased, the nitrogen protection effect is not good, and the feeding must be completely opened, and it is very difficult to remove the reaction mixture. Troublesome and difficult to solve the problem.
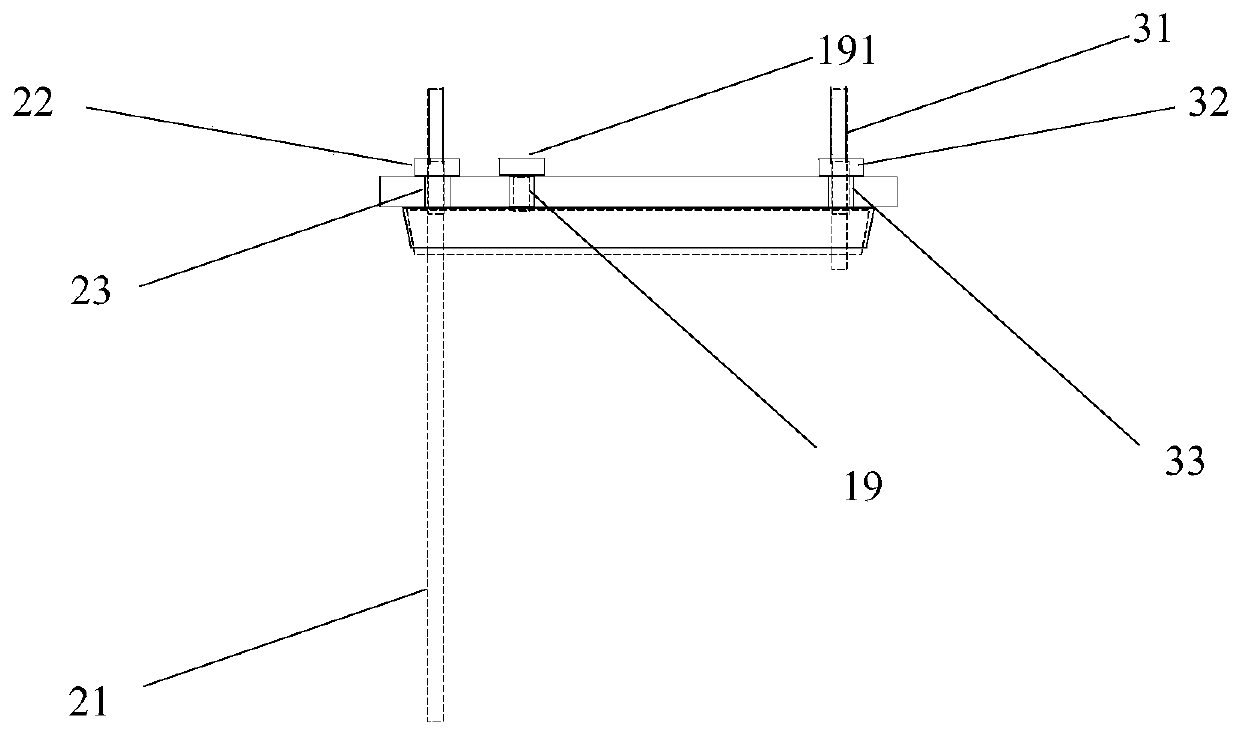
[0040] The center of kettle lid 11 has middle hole 14, and middle hole 14 is the circular through hole that the center is positioned at kettle lid 11 centers, and its inner side surface is frosted shape, is used for bonding with the peripheral surf...
Embodiment 2
[0051] 1) Get a reaction kettle as claimed in claim 2, clean and dry, fix the body 1 on the fixture 4, open the lid 11, add 1000ml of N,N-dimethylformamide, then add 50g of 8 -Bromoquinoline, and the cuprous cyanide of 33.5g and the cuprous iodide of 6g, close kettle lid 11, pass into nitrogen with 160ml / min, stir 10min with the rotating speed of 650 revs / min, slow down to 160 revs / min and keep it, raise the temperature to 110°C for 3h, cool the system to room temperature, open the lower outlet plug 132, pour the system mixture into 1350mL water to obtain A substance, place it in a large wide-mouth glass container, and use a total of 150ml of water Rinse the inside of the cylinder 13 once and combine it with the substance A, add 500mL ammonia water, extract the product 3 times with 500mL ethyl acetate, combine the organic phases, wash 3 times with 300mL saturated salt water, stir and dry with 50g of anhydrous sodium sulfate for 40min, pump Filter, concentrate and remove the s...
Embodiment 3
[0059] 1) Get a reaction kettle as claimed in claim 2, clean and dry, fix the body 1 on the fixture 4, open the lid 11, add 1050ml of N,N-dimethylformamide, then add 55g of 8 -Bromoquinoline, and the cuprous cyanide of 35g and the cuprous iodide of 6.5g, close kettle lid 11, pass into nitrogen with 140ml / min, stir 10min with the rotating speed of 630 revs / min, slow down to 150 revs / min and keep it, raise the temperature to 100°C for 3h, cool the system to room temperature, open the lower outlet plug 132, pour the system mixture into 1400mL water to obtain A substance, place it in a large wide-mouthed glass container, and use a total of 100ml of water Rinse the inside of the barrel 13 once and combine it with the substance A, add 500mL of ammonia water, extract the product 4 times with 500mL of ethyl acetate, combine the organic phases, wash twice with 300mL of saturated salt water, stir and dry with 50g of anhydrous sodium sulfate for 50min, pump Filter, concentrate and remov...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com